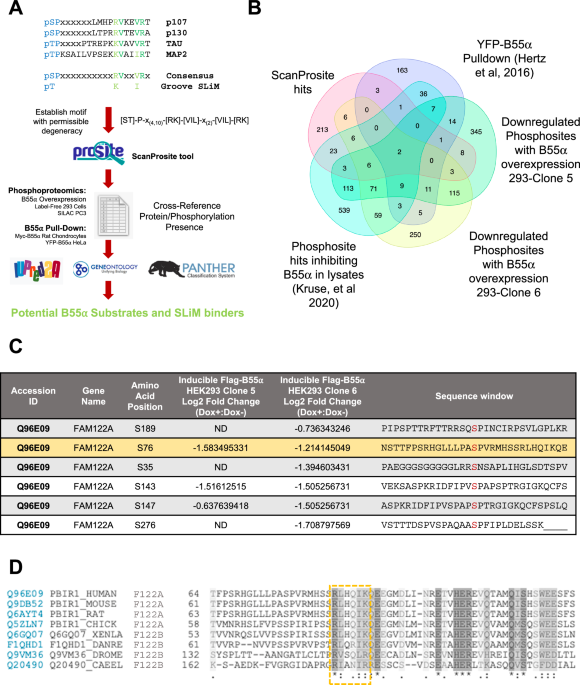
A filtered proteome-wide search identifies FAM122A as a potential SLiM-containing PP2A/B55α interactor
Using extensive mutational analysis and competition assays, we have previously defined the amino acid residues within a SLiM required for substrate binding to B55α. In the p107 model substrate, these residues direct the dephosphorylation of a proximal phosphosite. We have also shown that the SLiM is conserved in the unrelated substrate TAU and is required for its dephosphorylation. This allowed us to define an initial consensus B55 substrate SLiM, ‘p[ST]-P-x(4,10)-[RK]-V-x-x-[VI]-R’14. In this report, we have used a proteome-wide search tool, ScanProsite24,25, to identify potential novel PP2A/B55 substrates with a degenerate version of this SLiM (p[ST]-P-x(4,10)-[RK]-[VIL]-x-x-[VIL]-[RK]) that includes potential conservative amino acid variants (Fig. 1A, Supplementary Data 1). This search yielded 275 proteins (1.3% of the proteome). We then filtered the list of potential substrate candidates by determining which of these proteins have been detected by B55α pull-down proteomics10, identified in phosphoproteomic analyses where B55α activity was inhibited in lysates1, or in phosphoproteomic datasets of proteins dephosphorylated following doxycycline-inducible expression of FLAG-B55α in HEK293 cells (Supplementary Data 2).
A Steps in the pipeline towards the identification of PP2A/B55α substrates and other binders that use the SLiM. A generalized groove SLiM was derived from the similarity in p107, p130, TAU, and MAP2 sequences. This motif was permitted to have some degeneracy and variability in position from the amino-terminal phosphosite and searched within the human proteome using ScanProsite24,25. This list was cross-referenced to B55-related datasets and the proteins assessed for conservation (PANTHER52,53), gene ontology (using GENEONOTOLOGY54,55), and presence within IDRs (using IUPRed2A56). B Venn diagram showing common hits among the ScanProsite search and proteins in the indicated datasets (Supplementary Data 1 and Data 2). C Phosphosites downregulated upon doxycycline induction of B55α expression in HEK293-iB55α clones 5 and 6 in FAM122A, one of the common hits among the datasets. D The SLiM is conserved in bilateral animals: worms, flies, amphibians, birds, and mammals. The SLiM is boxed. “*” (asterisk) indicates positions that have a single, fully conserved residue, “:” (colon) indicates conservation between groups of strongly similar properties; “.” (period) indicates conservation between groups of weakly similar properties.
A recurring ‘hit’ among the datasets was FAM122A (Fig. 1B, Supplementary Data 1), also known as PABIR1 or PPP2R1A-PPP2R2A-interacting phosphatase regulator 1, a highly conserved protein of predicted disorder and a proposed inhibitor of the PP2A/B55α holoenzyme22. FAM122A exhibits a potential SLiM sequence, RLHQIK, was identified in at least two independent B55α pulldown datasets10,17, and exhibits several p-SP sites that are downregulated following upregulation of B55α in HEK293 and PC3 cells (Fig. 1C), including a proximal phospho-SP amino terminal from the potential SLiM sequence. Of note, a B55α pulldown dataset in Rat Chondrosarcoma cells, which we had previously reported, showed that FAM122A is the most abundant protein in B55α complexes other than the holoenzyme subunits17. Ontology analysis revealed that FAM122A originated by duplication of an ancestral FAM122B detected in bilateria present in worms, flies, frogs, and fish (Supplementary Fig. 1A). Amino acid sequence conservation shows that the SLiM sequence identified in FAM122A is conserved even in distant worms and flies (Fig. 1D; Supplementary Fig. 1B), which also express B55/PP2A holoenzyme subunits and in the two FAM122A paralogs, FAM122B and FAM122C. Altogether, these data suggest that FAM122A is a potential SLiM-dependent inhibitor.
FAM122A is a SLiM-dependent interaction partner of B55α
Since FAM122A has been reported to be a bona fide inhibitor of PP2A/B55α22, we determined if its interaction with B55α is dependent on functional SLiM sequences via immunoprecipitation of Myc-B55α and FLAG-FAM122A tagged mutants transfected in HEK293T cells. Mutation of murine mFLAG-FAM122A R81/L82 or I85/K86 SLiM residues to Ala abolished binding to Myc-B55α in reciprocal immunoprecipitations (Fig. 2A). In contrast, an S73A mutation, abolishing a phosphosite amino-terminal to the SLiM, had no effect (Fig. 2A, Supplementary Fig. 2A). Consistent with a functional SLiM, the B55α D197K mutant, which does not bind B55α substrates14, also failed to bind FLAG-FAM122A (Fig. 2A). D197 is located in a deep groove on the surface of B55α, adjacent to the active site of the catalytic subunit. To rule out the possibility of the positively charged residues solely being responsible for the interaction, non-polar residues, Leu and Ile, were individually mutated to Ala in a GST-FAM122A deletion construct and subject to a GST-pulldown assay using HEK293T lysates. As shown in Fig. 2B, both L82 and I85 are critical for binding the holoenzyme in vitro. However, a short FAM122AL70-K86 peptide fused to GST containing the ‘RLHQIK’ SLiM was not sufficient to bind the holoenzyme in vitro (Supplementary Fig. 2B), suggesting that additional residues contribute to binding. Thus, we next determined if other conserved regions in FAM122 family members (FR) were required or contributed to binding. FRs were selected by aligning the human FAM122A paralogs, FAM122B and FAM122C and denoting regions of conservation (Fig. 2C). Figure 2D schematically details the GST constructs used in pull-down assays of HEK293T lysates. Conserved region FR4, which contains the SLiM, is sufficient for binding, while regions FR1, FR2, and FR3, fail to bind B55α, but FR2 and FR3 significantly increase the binding avidity of FR4 (Fig. 2D–F). As charge-charge dynamic interactions in PP2A/B56 substrates increase binding avidity to B5626, we mutated conserved positive residues in vertebrate FAM122A. Mutation of these conserved Arginine residues in FR2, FR3, and FR4 reduced binding to B55α (Supplementary Fig. 2C). Taken together, the interface of FAM122A:B55α binding is mediated via the SLiM and enhanced, at least in part by additional interactions of charged residues in FAM122A.
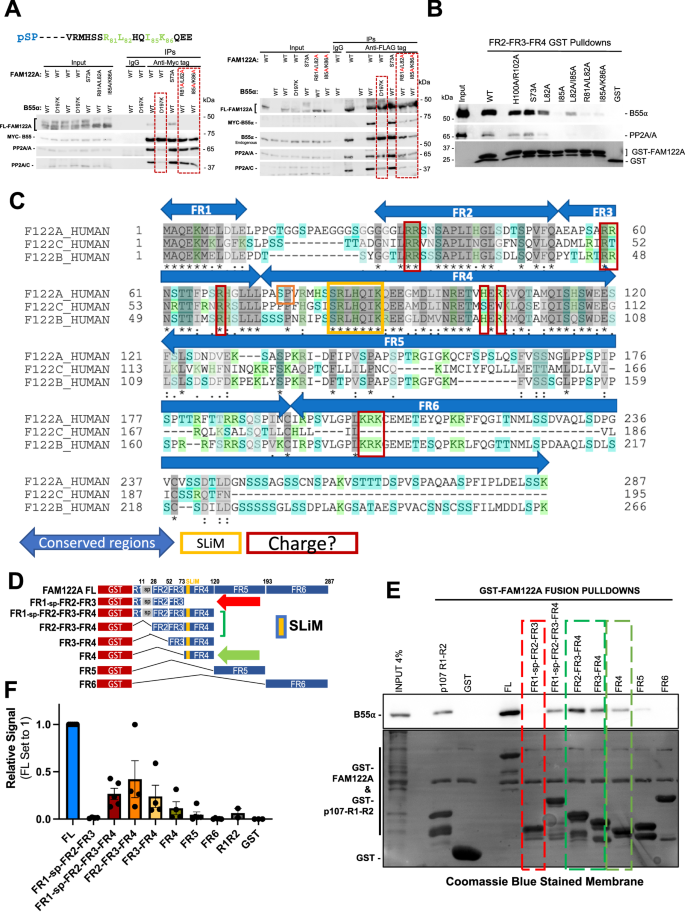
A HEK293T cells were cotransfected with FLAG-FAM122A and Myc-B55α WT and MTs. Anti-Myc and -FLAG IPs were analyzed by western blot (n = 3). B Glutathione beads loaded with WT and MT GST-FAM122A deletion constructs were incubated with purified PP2A/B55α and the pulldowns were analyzed by western blot, demonstrating dependence on the FAM122A SLiM residues for B55α binding. C Human FAM122 family amino acid sequence alignment (FAM122A: Q96E09, FAM122C: Q6P4D5, and FAM122B: Q7Z309-3) using the UniProt sequence alignment tool. Regions were selected based on amino acid conservation; the SLiM and residues that could mediate dynamic charge-charge interactions are marked. D–F GST-FAM122A pulldown assays with the indicated constructs were done as in B and quantitated (F). The following are presented as biological replicates: FL, FR1-sp-FR2-FR3-FR4, FR5, FR6 n = 5. FR1-sp-FR2-FR3, FR2-FR3-FR4, FR3-FR4, FR4 n = 4. GST n = 3. R1R2 n = 2. Data are presented as mean values +/− SEM. Source data are provided in the Source Data file.
AlphaFold2 predicts that the hFAM122A SLiM folds as a short helix, followed by a longer C-terminal helix, and that R84 (corresponding to murine R81), contacts B55α D197
Despite previous computational metrics denoting disorder in FAM122A, AlphaFold2 (AF2), an artificial intelligence (AI) program that performs predictions of protein structure27, predicts a helix in FAM122A consisting of residues R84-G93 that encompasses the SLiM. This is followed by a C-terminal helixL95-S120 (scoring confident to very high confident prediction, Fig. 3A). The rest of FAM122A was predicted to be predominantly disordered. We then used the AF2 implementation in ColabFold28 and AlphaFold-Multimer (AFM) to predict structures of the complexes of B55α and FAM122A and the entire complex of B55α, PP2A, the scaffold subunit, and FAM122A. Without the use of templates, AF2 in ColabFold predicted the beta-propeller folding of B55α with high accuracy compared to the experimentally determined structure (1.8 Å RMSD to PDB:3dw8, chain B). Four of five models placed the predicted short helix of FAM122A at the mouth of the B55α top groove, with the side chain of R84 forming a salt bridge (at 2.9 Å) with the side chain of B55α D197 (Fig. 3B, C, Supplementary Fig. 3A). In the model, L85 and L88 of FAM122A make hydrophobic contacts with B55α L225 (Fig. 3B, C). Consistently B55αD197K and B55αL225A fail to bind FAM122A (Fig. 3D). To demonstrate that the helical structure containing the SLiM residues is critical for binding to B55α, we determined the effect of mutations likely to disrupt/alter α-helices (Pro and Gly) vs. mutations that are typically tolerated (Ala). The mutations were introduced in positions 3 and 4 of the FAM122A SLiM (RLHQIK), as substitution to Ala in the p107 SLiM at these positions does not affect binding. AF2 predictions were consistent with this idea, as the RLAAIK FAM122A variant formed a helix with “pLDDT” accuracy values comparable to WT FAM122A (Supplementary Fig. 3B). As predicted, mutation to Ala (RLAAIK) had no effect on binding (Fig. 3E). In contrast, FLAG-FAM122A variants with α-helix breaker residues (RLPPIK) or (RLGGIK) failed to bind Myc-B55α in anti-FLAG immunoprecipitations of lysates of HEK293T cells (Fig. 3E). Consistently, AF2 either does not bind these peptides to B55α or scores them poorly (Supplementary Fig. 3B).
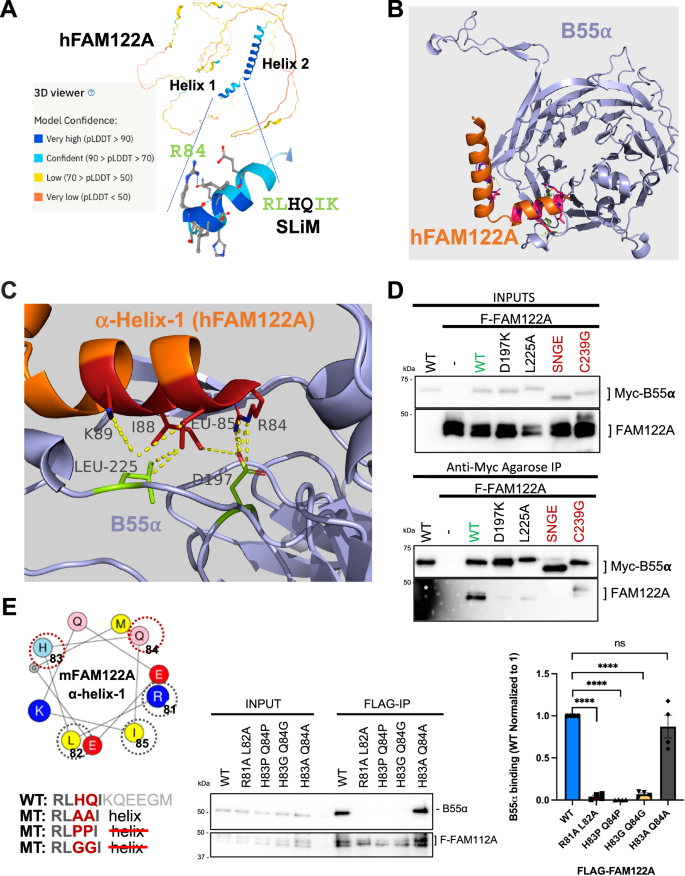
A AlphaFold2 predicts the folding of two adjacent hFAM122A helices, with helix 1 containing the SLiM. B AlphaFold2_advanced predicts that helix one binds at the mouth of a deep groove on the top of B55α that we have previously shown interacts with B55α substrates. C A closeup of the model aligned to the holoenzyme shows hFAM122A R84 making contacts with D197 in B55α and L85-I88 with potential contacts to B55α L225. The position of helix-2 suggests a mechanism for blocking access to the active site and/or potential stabilization of the enzyme/inhibitor complex. Residues are shown as numbered sticks: red for hFAM122A, and lime for B55α. D anti-Myc IPs of HEK293T co-transfections of WT FLAG-FAM122A with Myc-B55α and indicated mutants (n = 3). E mFAM122A α-helix-1 representation using the HeliQuest tool. The amino acids that contact B55α are marked by a grey dashed circle. The variable residues mutated to Pro or Gly to break the helix are marked by a red-dashed circle. HEK293T cells were transfected with FLAG-mFAM122A WT and helix tolerant and breaker MTs. Anti-FLAG IPs were analyzed for binding to endogenous B55α by western blot and relative binding was quantitated (n = 4 biological replicates). Relevant proteins are indicated. Data was analyzed for statistical significance using a One-way ANOVA (two-sided) with Dunnett correction for multiple comparisons. WT: R81A L82A p-value < 0.0001. WT: H83P Q84P p-value < 0.0001. WT:H83G Q84G p-value < 0.0001. WT:H83A Q84A p-value = 0.4076 (ns). Data are presented as mean values +/− SEM. Source data are provided in the Source Data file.
FAM122A helix-2 inhibits substrate dephosphorylation through predicted interactions of hFAM12A E100 and E104 with the active site of PP2A/C
Of note, the C-terminal long helix is predicted to be placed at a ~90° angle (Fig. 3B). Superposition of the heterodimer of B55α and FAM122A with the experimental structure of the PP2A-B55α-Scaffold complex (PDB 3dw813) suggested that it could fill the space between B55α and the active site of PP2A/C (Fig. 4A, only model 1 is shown). This strongly suggests that FAM122A would block substrate access to the PP2A/C active site. This was confirmed when a retrained AFM v2.3 was made available in December 2022. AFM v2.3 produced a model of the full tetrameric complex containing FAM122A (Fig. 4B, Supplementary Fig. 4A). A complete surface model of the PP2A/B55α holoenzyme bound to FAM122A predicts that FAM122A fills the space between the active site of PP2AC and B55α with contacts to both proteins (Fig. 4B). Moreover, residues within the long helix are predicted to be in close proximity with PP2A/C residues, suggesting potential additional contacts that could stabilize holoenzyme/inhibitor binding. To gain insight into this possibility, we compared FLAG-FAM122A binding to wildtype (WT) Myc-B55α or a point mutant (C239G) that dramatically reduces B55α binding to the PP2A/A scaffold, rendering B55α monomeric in cells, yet capable of binding p107 and potential B55α substrates (Supplementary Fig. 4B). This mutation reduced binding to FAM122A and resulted in a shift in mobility that could indicate changes in phosphorylation (Fig. 3D). In addition, immunoprecipitation from lysates of cells transfected with this B55α mutant vs WT followed by mass spec shows FAM122A as the most enriched holoenzyme binder (Fig. 4C, Supplementary Data 3). This indicates that FAM122A makes contacts with other subunits in the holoenzyme. Figure 4D shows that hFAM122A E100 and E104 are predicted to make contacts with the manganese ions in the active site and the completely conserved residues in PP2A/C that coordinate substrate phosphate (R89, H118, and R214)9. Consistently, a mFAM122A variant with the residues corresponding to hFAM122A E100 and E104 mutated to Ala or Pro, lack inhibitory activity towards the pDiFMUP substrate (Fig. 4E), but retain binding to B55α through helix1, even when the corresponding E residues in mFAM122A are changed to a positive charge (E to K reversals, Supplementary Fig. 4C). Altogether these data show that helix-2 acts as the inhibitory helix, whereas helix-1 mediates FAM122 A binding to the holoenzyme through contacts in the B55α top groove.

A Superimposition of the AF2 B55α:hFAM122A model aligned to the structure of the holoenzyme (3DW8) using the B55α subunits in each structure positioning helix-2 of hFAM122A near the active site of PP2A/C. B AlphaFold-Multimer v2.3 Surface model of the PP2A/B55α holoenzyme bound to hFAM122A76-122. C Pulldown IP mass spec analysis of Myc-B55α-C239G (renders B55α monomeric) vs Myc-B55α-wildtype (holoenzyme) demonstrating FAM122A preferentially binds the holoenzyme. Statistical significance was determined using a two-sided student’s t-test (no multiple comparisons). Other relevant proteins are indicated (Supplementary Data 3). D AlphaFold-Multimer v2.3 model closeup of hFAM122A and the three subunits of the PP2A/B55α holoenzyme. Distances for the predicted contacts between residues in hFAM122A with B55α and PP2A/C are indicated. The distance of E100 and E104 to the Mn++ metals is also indicated. E DiFMUP phosphatase assay (15 min duration) of purified PP2A/B55α holoenzyme preincubated with purified full-length HA-tagged wild-type or helix-2 FAM122A mutants of residues predicted to promote holoenzyme inhibition. Each reaction was performed in triplicate with a n = 3 and statistical significance analyzed using a One-way ANOVA (two-sided) with Dunnett correction for multiple comparisons. PP2A/B55: + FAM122A WT p-value < 0.0001. PP2A/B55: + FAM122A E97P E101P p-value = 0.0706 (ns). PP2A/B55: + FAM122A E97A E101A p-value = 0.2067 (ns) PP2A/B55 + BSA p-value = 0.4483 (ns). Data are presented as mean values +/− SD. Source data are provided in the Source Data file.
FAM122A abrogates p107 binding to B55α in vitro and in cells
A previous report suggested that FAM122A inhibits PP2A/B55α by a mechanism involving degradation of the PP2A/C catalytic subunit22. However, we have not seen any effect in the catalytic subunit of the B55α holoenzyme when we co-express FAM122A in cells (Fig. 2A). In contrast, we have observed that under comparable concentrations of FAM122A and p107, FAM122A pulls down more B55α in vitro (Fig. 2E, Supplementary Fig. 2B), suggesting comparatively higher affinity. In agreement with this, purified HA-FAM122A strongly inhibits B55α:p107 binding in vitro at FAM122A concentrations comparable to the PP2A/B55α complex (nM range) and with vast excess of p107585-691 (μM range) (Fig. 5A, B) in in vitro competition assays. Additionally, a dose-dependent increase in transfected FLAG-FAM122A in HEK293T cells displaces FLAG-p107 from Myc-B55α (Fig. 5C). At comparable FLAG antibody signals, FAM122A abolishes p107 binding, which is consistent with FAM122A using its SLiM to block substrate binding and other conserved residues to bind with higher affinity than B55α substrates (among all PP2A/B55α substrates tested by us, p107 binds the tightest to B55α). Further supporting a SLiM competition model, AF2 also predicts a helix spanning the p107 R621VKEVR SLiM sequence, which prompted our use AFM29 to predict the interaction of p107 residues 612-648 with B55α. The original AlphaFold-Multimer was trained on multi-protein complexes in the Protein Data Bank deposited before April 2018. The model shows a helix formed from residues 620-633 which exactly superimposes the SLiM of p107 with that of FAM122A, with interaction of R621 with D197 and V625 with L225 (Fig. 5D). This binding model is compatible with the placement of p107 P-S640, located C-terminally with respect to the SLiM, at the PP2A/C active site. We therefore sought to determine if dephosphorylation of S640 was, like S615, dependent on the SLiM using p107-R1-R2 mutant variants that only contain a single SP-phosphosite. To make the phospho-S640 recognizable by an anti-p-SP antibody, we mutated the residue preceding S640 to Met. Figure 5E shows that S640 is dephosphorylated with kinetics comparable to S615, and that dephosphorylation of both phosphosites is dependent on the SLiM. Figure 5F schematically summarizes the FAM122A competitive mechanism of inhibition. Finally, we determined the approximate affinity of FAM122A and p107-R1-R2 for B55α at the interaction equilibrium using a previously described depletion assay30. The KD for GST-FAM122A/B55α was ~2.44 fold lower than that of GST-p107-R1-R2:B55α (Supplementary Fig. 5A, B), which is consistent with FAM122A effectively competing with p107 for binding to B55α in vitro and in cells.
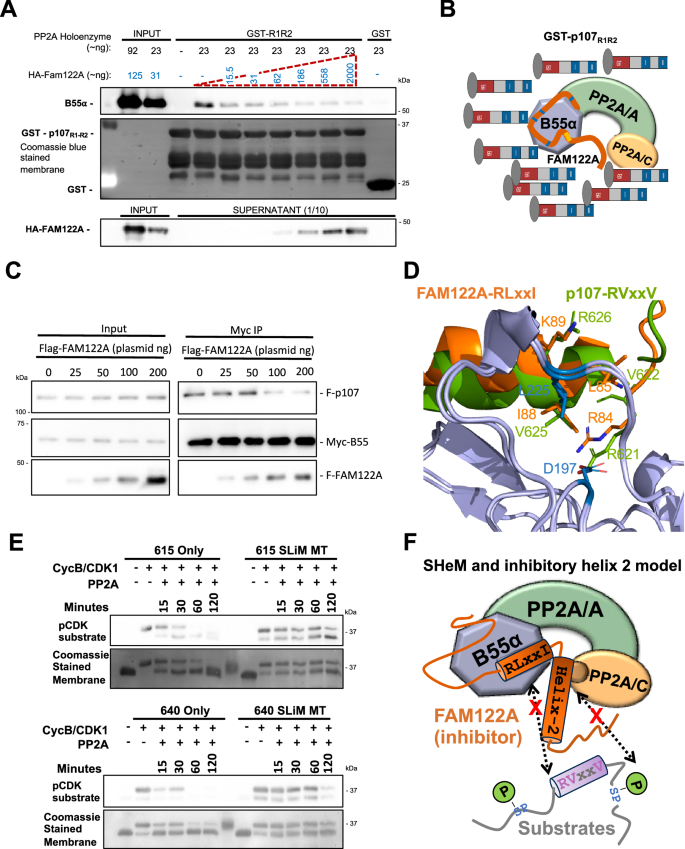
A Purified PP2A/B55α holoenzymes were incubated with purified HA-FAM122A (expressed in E coli) starting with the same concentration as B55α in each sample up to a ~100X. B55α: R1R2 binding in GST-p107-R1R2 pull-downs was inhibited at the lowest FAM122A concentration. (n = 3). Source data are provided in the Source Data file. B Schematic interpretation of A. C Co-transfection of invariable amounts of Myc-B55α and FLAG-p107 plasmids with increased amounts of FAM122A, as indicated. (n = 3). Source data are provided in the Source Data file. Anti-Myc immunoprecipitates were analyzed by western blot and relevant proteins are indicated. D AlphaFold-Multimer model of superimposing a predicted helix containing the p107 with the predicted helix-1 of hFAM122A. Conserved SLiM residues in both proteins superimpose and make contacts with D197 and L225. Residue numbering corresponds to the human proteins. E GST-R1R2 constructs created with a single phosphosite (S615 or S640). Arginine to Alanine mutations disrupted the SLiM motif in the indicated constructs. Western blot visualization of time-dependent kinase-dephosphorylation assays of the R1R2 constructs. GST-R1R2 variant beads were phosphorylated with CDK1/cyclin B kinase. Washed phosphorylated beads were incubated with PP2A/B55α for the indicated times (n = 3). F Schematic representation of the substrate-competitive SHeM mechanism of inhibition, which involves the two helices. Helix-1 (SHeM) mediates binding to B55α and helix-2 acts as inhibitory flapper by directly contacting the active site.
FAM122A promotes proliferation
We next investigated the functional role of FAM122A in the cell cycle using CRISPR knockouts. FAM122A knockout in HEK293 cells dramatically reduces colony formation and proliferation (Fig. 6A–C). Inhibition of proliferation was also observed in T98G and U-2 OS cells using 2 different sgRNAs (Fig. 6C, Supplementary Fig. 6A). Reconstitution of GFP-FAM122A in HEK293-FAM122A-KO cells partially rescued the proliferation defect in a SLiM-dependent manner (Fig. 6D, Supplementary Fig. 6B), indicating that the defects on FAM122A are at least partially dependent on PP2A/B55α inactivation. This is further supported as FAM122A knockout in HEK293 cells results in increased phosphatase activity relative to wildtype control in dephosphorylation of substrates phosphorylated on S/T-P sites (Supplementary Fig. 6C).
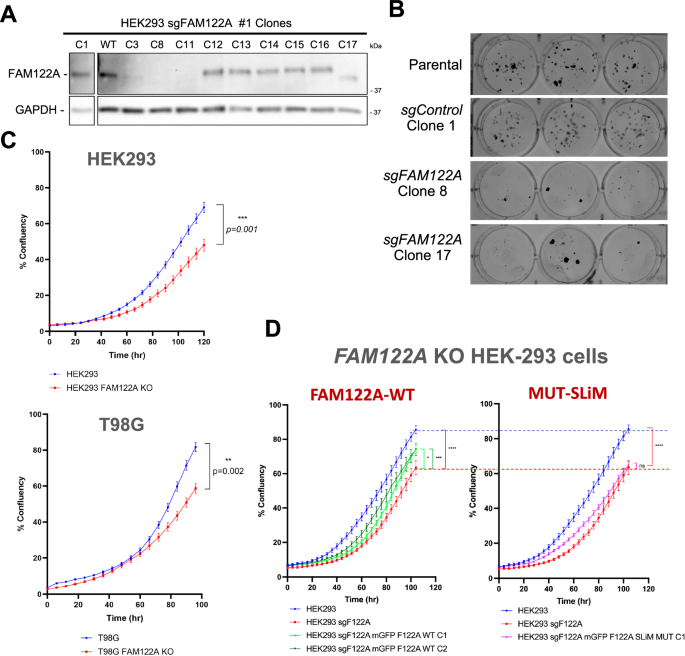
A Western blot analysis of FAM122A CRISPR HEK293 clones (the C8 KO and C17 truncation were selected for further analysis) (n = 3) B Colony formation assays of control (parental and C1) and KOs (C8 and C17). C Ablation of FAM122A in HEK293, T98G cells inhibits proliferation. Cells were seeded in triplicate and the percent confluence was imaged with an Incucyte SX5. Statistical analyses of proliferation curves comparing parental cells to FAM122A knockout cell lines was conducted using a Wilcoxon matched-pairs signed rank test (two-sided). Data are presented as mean values +/− SEM. D A cassette directing the expression of FAM122A WT and SLiM-MT was stably introduced in HEK293 FAM122A KO cells. Expression of FAM122A wildtype but not SLiM mutant rescued the proliferation defect. Statistical analyses of proliferation curves comparing rescues to FAM122A knockout cell lines was conducted using a Friedman’s test (two-sided) of the last 10 measurements. HEK293 vs KO p value < 0.0001 (****), HEK293 WT Clone 1 p value = 0.0436 (*), HEK293 WT Clone 2 p value = 0.0002 (***), HEK293 SLiM MUT Clone 1 p value = 0.2640 (ns). FAM122A WT and SLiM mutant reconstitution are show in separate graphs compared to the same controls to more clearly visualize partial rescue (green curves) vs. absence of rescue with the SLiM mutant (magenta curve). Data are presented as mean values +/− SEM. Source data are provided as a Source Data file.
FAM122A is required for timely cell cycle entry and progression through the G1 phase of the cell cycle following mitogen stimulation
Because PP2A/B55α opposes CDK inactivation of the pocket proteins, pRB, p107, and p13017,18,19,31, and we have shown that PP2A/B55α directly dephosphorylates p107 in vitro and in cells14, we determined if knockout of FAM122A had any effects in cell cycle re-entry and progression through the cell cycle of T98G cells in response to serum stimulation. Parental and FAM122A KO T98G cells were serum starved for 3 days and then stimulated with serum. Cells were collected at the indicated points for DNA content FACS cell cycle and western blot analysis. FACS analysis shows a >4 h delay in the cells progressing from G0 to mitosis (Fig. 7A, nocodazole was added 16 h post-release to prevent cells from entering the next cycle). To observe the delay more clearly, we performed a serum starvation and re-stimulation experiment where cells were collected hourly from 16 to 20 h. By 20 h the KO cells had not reached the number of cells in S phase already seen in the parental cells by 16 h (Fig. 7B and Supplementary Fig. 7B). Moreover, double staining with EdU and PI, showed an increase in the number of cells remaining in G1 at 12, 15 and 18 h. post serum stimulation in WT (65.3%, 38.6%, 26%) vs. KO cells (69.7%, 51.9%, 39%) (Fig. 7C, quantitated in Supplementary Fig. 7A). Western blot analysis showed a delay in the upregulation and reduced peak levels of Cyclin D1 that are consistent with the delays in pRB phosphorylation (a PP2/B55α substrate7,14,32), the expression of E2F-dependent gene products (p107, Cyclin A) and the degradation of p130, which is dependent on CDK2 activity (Fig. 7D). Similar delays in cell cycle progression and the expression of these markers were observed in cells where FAM122A was knockdown by siRNA (Supplementary Fig. 7C, D).

A FAM122A-WT and -KO T98G cells were serum starved for 60 h and re-stimulated with DMEM supplemented with 10% FBS and collected at the indicated time points and analyzed by PI/FACS. Nocodazole was added to prevent cells from progressing beyond mitosis. A >4 h delay is observed by the time the cells reach mitosis, but the delay is already noticeable at the G1/S border. B A serum starvation-restimulation experiment focused on the G1/S transition (1 h time points) that shows a delay in cells entering in S-phase. C T98G Parental and KO cells were serum-starved for 72 h and subject to serum-restimulation. 10 μM EdU was added 1 h prior to collection. The cells were fixed with 4% PFA in PBS, washed, and subject to saponin-based permeabilization followed by the Cy5-azide click-chemistry reaction and stained with PI/FACS analysis. The percentage of cells in G1 is indicated, suggesting delays prior to the onset of S-phase. D Delays in the expected modulation of pRB proteins and the expression of E2F-depedent gene products (Cyclins E and A and p107). The earliest defect is the limited expression of cyclin D1, whose expression is regulated by mitogens. Experiment performed in biological triplicate. E The effects of FAM122A KO in the activation/inactivation of key components of the major mitogenic signaling pathways regulating cyclin D1 expression in serum-starved and restimulated T98G during early signaling (AKT, ERKs, GSK3β and their relevant phosphoforms are indicated). Experiment performed in biological triplicate. Source data are provided as a Source Data file.
Because the decreased expression of cyclin D1 could explain a delay on pRB inactivation and passage to the restriction point and cyclin D1 expression is controlled by early mitogenic signaling33, we performed a serum starvation and re-stimulation experiment collecting cells at short time points preceding the expression of cyclin D1. Lysates of these cells revealed delays in the activation of ERKs and AKT, both upstream regulators of cyclin D1 (Fig. 7E). The magnitude of peak signaling was also clearly lower (Fig. 7E). ERKs control cyclin D1 transcription, while AKT controls the stability of Cyclin D1 by inactivating GSK3β, which is known to promote Cyclin D1 degradation33. Consistently, inactivation of GSK3β is delayed in FAM122A-KO cells (Fig. 7E). These results, together with the FAM122A SLiM-dependent defects in proliferation (Fig. 6), strongly indicate that FAM122A controls PP2A/B55α activities that attenuate early mitogenic signaling including AKT T30834, a site known to be directly regulated by PP2A/B55α, as well as other direct/indirect effects that oppose ERK activation (Supplementary Fig. 7E).
FAM122A is required for checkpoint activation in response to replication stress (RS)
Our data together with that published by others supports that FAM122A is an abundant regulated inhibitor of PP2A/B55α3. FAM122A has been recently shown to be phosphorylated and inhibited by CHK1 in NSCLC cell lines promoting activation of PP2A/B55α, which stabilizes WEE1 leading to inhibition of CDK123. Interestingly, ablation of FAM122A restores WEE1 expression and the basal levels of inactive CDK1, restores fork replication speed, and dramatically reduces RS and DNA damage, suggesting that maintaining downstream control of CDK1 through FAM122A is the main mechanism by which CHK1 ensures genomic stability in these cells23. However, our data are only partially consistent with this model. Analysis of cell cycle effects of ablation of FAM122A in HEK293 cells using BrdU incorporation and propidium staining assays shows a clear reduction in the incorporation of BrdU to DNA during S phase, without a decrease in the number of cells with PI staining corresponding to S phase cells (Fig. 8A, the mean fluorescence intensity is 4181 for the CRISPR control and 1662, for the FAM122A KO, n = 3). This suggests that cells are synthesizing DNA more slowly and that the decreased proliferation in these cells, which have a defective pRB pathway, could be the result of extension of S-phase length, rather than accumulation of cells in G1. Thus, we determined the effect of FAM122A KO in DNA-fiber length in exponentially growing HEK293 cells. DNA fiber length synthesis was decreased by ~18.8%, as determined by IdU and CIdU incorporation assays (Fig. 8B). These results show that elimination of FAM122A induces replication stress, which was not reported in NSCLC cell lines23. Thus, these cells are synthesizing DNA more slowly and their decreased proliferation likely results from an extension of S-phase length.
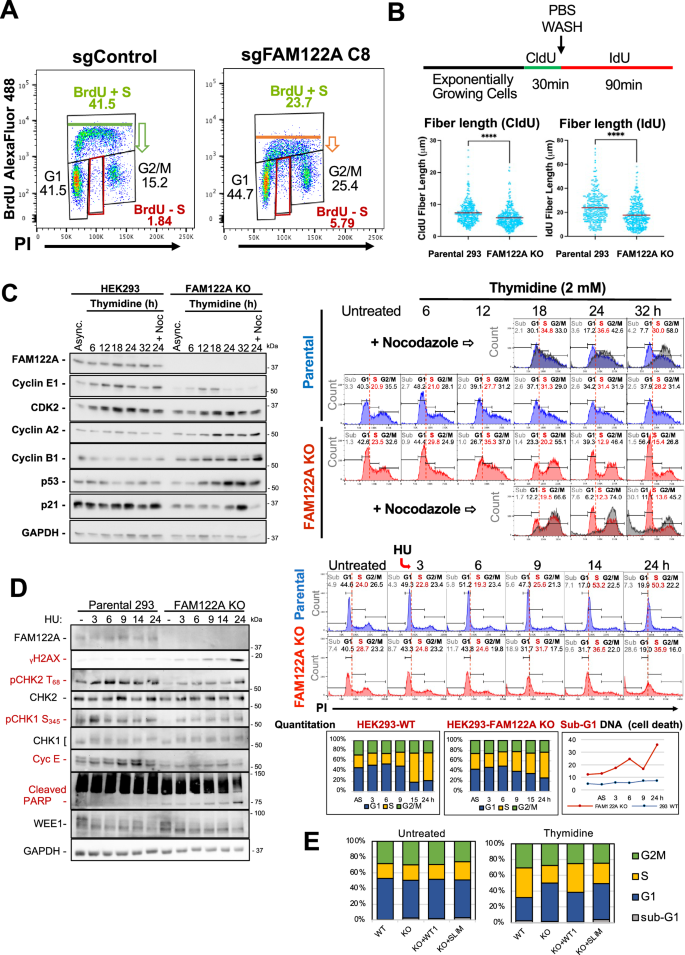
A Ablation of FAM122A KO in HEK293 cell results in a decrease in the overall incorporation of BrdU in cells in S phase and the appearance of a fraction of cells arrested in S-phase (BrdU negative, red boxes). B HEK293 Parental and FAM122A KO cells were subjected to CldU incorporation, washout, and IdU incorporation to measure DNA fiber length. FAM122A KO cell fiber length was shorter, demonstrating impaired DNA synthesis. Statistical significance was determined by Mann-Whitney U Test (non-parametric two-sided). Analyzed fibers were the result of three independent replicates. CldU Parental: KO p-value < 0.0001. IdU Parental:KO p-value < 0.0001. C HEK293 cells were treated with 2 mM thymidine for the indicated times and analyzed by PI flow and western blot using the indicated specific antibodies. These data demonstrate that FAM122A KO cells are refractory to arrest under thymidine nucleotide depletion at the G1/S border and in S-phase. Experiment performed in biological triplicate. D HEK293 cells were treated with 2 mM HU as indicated analyzed as in B. Attenuation of CHK2 and CHK1 phosphorylation indicates an ineffective checkpoint, which results in increased DNA damage (γH2AX accumulation) and cell death (increased PARP cleavage and increased sub-G1 DNA content). Experiment performed in biological triplicate. E Parental HEK293, FAM122A KO, and GFP-FAM122A WT and SLiM-MT reconstituted cells were challenged with 2 mM Thymidine for 24 h to initiate an S-phase arrest. The arrest was rescued in the WT-FAM122A expressing cells but not in SLiM-MT-FAM122A reconstituted cells.
HEK293 are synchronized by either single or double thymidine blocks (Supplementary Fig. 8A), which deplete endogenous nucleotides. In contrast, FAM122A KO HEK293 cells are refractory to synchronization at the G1/S transition, and thymidine washout resulted in massive cell death. Figure 8C shows a thymidine challenge that resulted in time-dependent accumulation of HEK293 cells at the G1/S transition and in S phase that correlated with accumulation of Cyclin E peaking at 18 h. By contrast, FAM122A-KO cells exhibited diminished sensitivity to nucleotide depletion and although they slowed down through S phase, they continued to progress through G2 and completed mitosis. Completion of mitosis in FAM122A KO cells was demonstrated by adding nocodazole to replicate samples 12 hours post washout, which resulted in massive accumulation of cells in G2/M with very few cells remaining in S phase by 24 h (Fig. 8C, note gray overlay over the untreated KO cells). This was followed by massive cell death (Sub-G1 DNA-accumulation). Progression of KO cells though S/G2/M was also clear from the accumulation of cyclins A and B that peaked at 18 and 24 h respectively. We also noted time dependent accumulation of p53, which started as cells were moving to G2/M and was followed by accumulation of p21 in G1 (p21 levels increase at 24 and 32 h, but this increase is blocked by nocodazole, indicating that p21 expression is upregulated in late mitosis or G1 (Fig. 8C). To confirm the diminished sensitivity to nucleotide depletion we used hydroxyurea (HU), which results in rapid and complete inhibition of DNA synthesis at a 2 mM concentration. Both the parental and FAM122A KO cells arrest in S-phase due to rapid nucleotide depletion, as the G1 centered peak moves to the right of the G1/S border delimited by a dashed red line. However, while WT cells arrest in S phase and are viable, the FAM122A KO cells die. Since the cells in the presence of HU are either at the G1/S border unable to initiate DNA synthesis or arrested in S phase, the FAM122A KO PI/FACS data shows that these S phase arrested cells are unable to survive. Consistent with these findings, the G1/S and intra-S phase arrest in parental HEK293 cells resulted in rapid check point activation as demonstrated by activation of CHK2 (P-T68) and CHK1 (P-S345) and time-dependent accumulation of cyclin E in parental cells. Checkpoint activation was largely attenuated in FAM122A-KO cells, which exhibited rapid and time-dependent accumulation of γH2AX, sub-G1 DNA, and potent attenuation of both CHK2 and CHK1 activation (Fig. 8D). Of note, we did not observe changes in WEE1 expression, indicating that the intra-S phase arrest mediated by FAM122A upon nucleotide deprivation is likely independent of WEE1. Altogether, these data strongly suggest that FAM122A controls PP2A/B55α-dependent steps upstream of these kinases. This is consistent with the ability of PP2A/B55α to negatively regulate ATM21, but likely involves other factors/substrates in this pathway (Supplementary Fig. 8G).
We next determined if the abrogation of the thymidine arrest checkpoint in HEK293 FAM122A KO cells could be rescued by reconstitution of FAM122A. Figure 8E shows that HEK293 FAM122A KO cells fail to arrest in S phase following 24 h incubation with 2 mM thymidine. Reconstitution of WT FAM122A, but not FAM122A SLiM-mutant, restored the arrest in S phase (Fig. 8E and Supplementary Fig. 8I).
It has been recently reported that inhibition of CHK1 reduces phosphorylation of FAM122A presumably on S37, increases binding to B55α and reduces binding of 14-3-3 proteins, which results in FAM122A upregulation in the nuclear fraction of A549 lung cancer cells23. FAM122A is also phosphorylated in cells at multiple sites that are consensus for the CDKs and ERK3, indicating a potential regulation during the cell cycle. Therefore, we determined if FAM122A interaction with B55α is regulated during the cell cycle or in response to checkpoint activation. We did not observe changes in the levels of B55α bound to tagged FAM122A when comparing exponentially growing HEK293 and T98G cells, to cells arrested at the G1/S transition with thymidine, the G2/M transition with the CDK1 inhibitor (RO3306), and in pseudo-metaphase with nocodazole (Supplementary Fig. 8B, C). Also, we did not observe regulation of these complexes in T98G cells progressing through the cell cycle from quiescence or exiting mitosis and progressing through G1 from a nocodazole arrest (Supplementary Fig. 8D, E). These data suggest that the overall amount of FAM122A/B55α complex is constant under all these conditions and that regulation may depend on the colocalization of the complex and substrates and dynamic relative changes in FAM122A/substrate affinity for B55α.
We also determined if HU and/or inhibition of CHK1, which cause strong replication stress promote changes in the FAM122A/B55α interaction and binding to 14-3-3 proteins, which have been proposed to sequester FAM122A in response CHK1 phosphorylation23. We did not detect changes in the B55α/FAM122A interaction in GFP-FAM122A transfected cells (Supplementary Fig. 8F). Surprisingly, we also did not detect formation of a FAM122A/14-3-3 protein complex upon induction of replication stress (Supplementary Fig. 8F). Therefore, FAM122A is critical to establish checkpoints in response to replication stress that modulate CHK1 and CHK2 signaling.
Given the apparent lack of regulation of the FAM122A/B55α interaction as determined via immunoprecipitation, we determined the localization of FAM122A during the cell cycle. Transiently co-transfected RFP-FAM122A with EGFP-h-H2B in HEK293T cells revealed that FAM122A is largely, if not exclusively, localized in the nucleus in interphase, and it is released in the cytoplasm in prometaphase following nuclear envelop breakdown (NEB) (Fig. 9A). Co-transfection of RFP-FAM122A, BFP-B55α and EGFP-h-H2B in 293 T cells reveals that a fraction of B55α localizes in the nucleus and that both FAM122A and B55α are excluded from condensed chromosomes in mitosis (Fig. 9B). To demonstrate that B55α and FAM122A form a physical complex in the nucleus we used a Split-FAST approach35. B55α-N-FAST and FAM122A-C-FAST10 vectors were co-transfected in U-2 OS cells and the FAM122A/B55α nuclear complex was detected upon incubation with the HMBR fluorogen (Fig. 9C). Moreover, we used immunofluorescence to determine the localization of both FAM122A and B55α in the interphase and mitosis of U-2 OS cells. As in HEK293T cells, FAM122A is nuclear in interphase, but it is not bound to chromatin in mitosis. A large fraction of B55α is also found in the interphase nucleus, and not bound to chromatin upon NEB. Of note, in late anaphase, both FAM122A and B55α appear to be recruited to chromatin (Supplementary Fig. 9A). Consistent with the nuclear localization of FAM122A, we identified a conserved nuclear localization signal (NLS, 201RKK), that upon mutation (201RKK to AAK) prevented FAM122A from reaching the nucleus (Supplementary Fig. 9B). Altogether these data show that FAM122A and B55α interact in the nucleus and that during mitosis, chromatin is devoid of FAM122A and B55α.
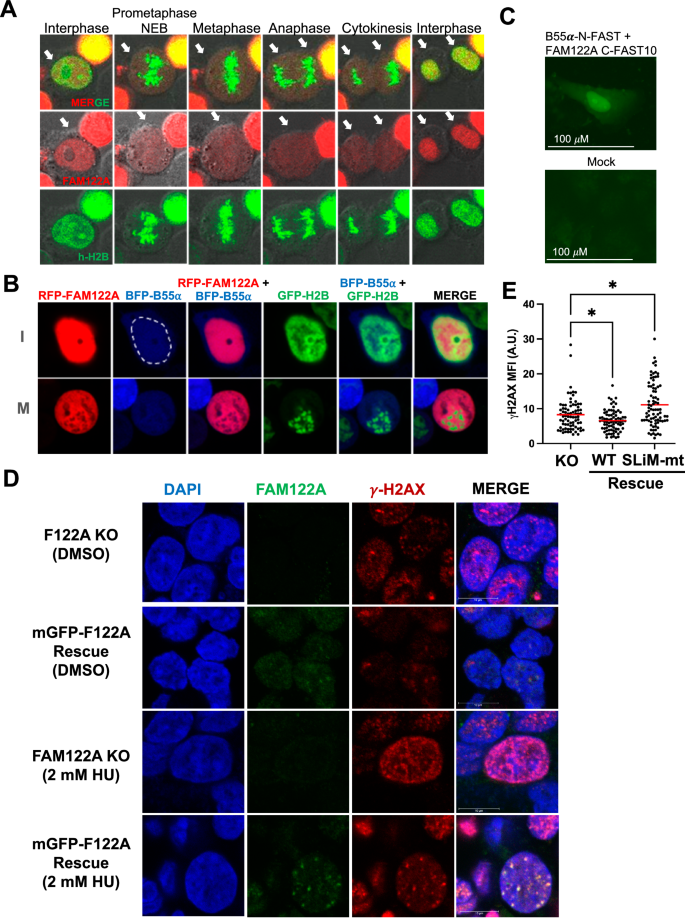
A mRFP-FAM122A wild-type was co-transfected with GFP-h-H2B into HEK293T cells and observed by time-lapse confocal microscopy (20x objective, 2.5x zoom factor). Arrows indicate the cell(s) in the cell cycle phase. B mRFP-FAM122A, BFP-B55α and GFP-h-H2B expressing vectors were transfected into HEK293T cells to monitor localization. Close up of two cells in interphase (I) and in Mitosis (M) are shown (the nuclear envelope is traced with a dashed white line to demonstrate expression of B55α in both the cytoplasm and the nucleus). C U-2 OS cells were transfected B55α and FAM122A SplitFast constructs and colocalization was visualized by adding the HMBR fluorogen to the media 48 h later. Experiment performed as biological triplicate. D FAM122A KO and reconstituted WT expressing C1 HEK293 cells were treated with 2 mM HU for 24 h, stripped of cytoplasmic proteins and fixed for immunofluorescence imaging to observe localization of FAM122A bound to chromatin in the context of γH2AX foci on a confocal microscope using a 63x objective and 5x zoom factor. The colocalization of FAM122A with γH2AX foci are represented by the yellow signal. Experiment performed as biological triplicate. E FAM122A KO and FAM122A reconstituted HEK293 cells (WT and SLiM MT) were treated with 2 mM HU for 3 h and subjected to gamma γH2AX immunofluorescence where mean-fluorescent intensity was analyzed using a Kruskal-Wallis test (two-sided) with a Dunn’s multiple comparison test. KO:WT Recon. p-value = 0.0429. KO:SLiM MT Recon. p-value = 0.0141. n = 3 biological replicates. Source data are provided as a Source Data file.
Given the apparent lack of global regulation of the B55α/PP2A complex in response to replication stress, we tested the hypothesis that FAM122A localization to chromatin is regulated in response to replication stress. HEK293 FAM122A-KO with and without FAM122A reconstitution were treated with HU for 24 hours in glass chambers and soluble nuclear proteins were extracted with cytoskeletal buffer36. DMSO-treated cells exhibit occasional γ-H2AX foci likely due to endogenous replication stress. Treatment with HU results in extensive DNA damage in the absence of FAM122A. Reconstitution of FAM122A in untreated cells results in clear localization of FAM122A at the chromatin and the larger foci colocalize with γ-H2AX. Treatment with HU resulted in strong recruitment of FAM122A to the sites of DNA damage (Fig. 9D). We next determined the effect of FAM122A reconstitution in γH2AX in HEK293 FAM122A-KO cells treated with HU for 3 h. As expected, FAM122A suppressed the γH2AX signal in a SLiM-dependent manner (Fig. 9E, Supplementary Fig. 9C). Therefore, these data shows that FAM122A subnuclear localization is regulated and that the relatively small fraction of FAM122A that is recruited to DNA-damage foci might be critical for the establishment of the checkpoint and suppression of DNA damage accumulation.