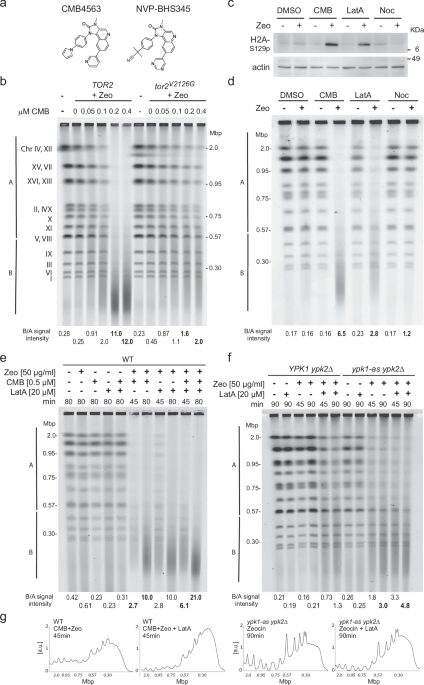
TORC2 inhibition triggers YCS after low-dosage Zeocin incubation
A chemicogenetic screen in yeast showed that TORC1 and TORC2 inhibition by NVP-BHS345 (hereafter BHS, Fig. 1a) was synthetic lethal in the presence of low levels of the radiomimetic chemical Zeocin or γ-irradiation34. This TOR inhibitor does not generate abasic sites, or ss breaks on its own, nor does it augment the level of oxidized base damage generated by a fixed concentration of Zeocin35. Instead, TORC2 inhibition leads to the conversion of oxidized bases into irreparable DSBs, triggering anywhere from 20 to 120 genomic DSB after a 1 h exposure of 50 to 80 μg/ml Zeocin34. These DSBs are monitored by neutral (non-denaturing) pulse-field gel electrophoresis (PFGE), which allows us to follow the conversion of full-length chromosome bands into chromosomal fragments of 50–200 kb in length (Fig. 1b). Here we identified a BHS-related imidazoquinoline called CMB4563 (CMB, Fig. 1a), that is roughly ten-fold more potent than BHS in the YCS assay35. We use the two inhibitors interchangeably, albeit at different concentrations.
a Structures of related imidazoquinoline TORC inhibitors CMB4563(CMB) and NVP-BHS345 (BHS). b Isogenic GA-6148 (TOR2) and GA-6150 (tor2V2126G) strains were treated with 75 μg/ml Zeocin (Zeo) with the indicated concentrations of CMB for 75 min. Genomic DNA was subjected to CHEF gel analysis and stained by EtBr, and quantitation is as described in Methods. The tor2V2126G mutation blocks the imidazoquinoline binding pocket rendering GA-6150 resistant to CMB. Molecular weight markers and yeast chromosome numbers are indicated. B/A signal intensity is used to compare chromosome fragmentation within one gel. In bold are values discussed in the text. c Exponentially growing GA-1981 (WT) yeast cells were treated with either 1% DMSO, 0.5 μM CMB, 20 μM Latrunculin A (LatA), or 50 μM Nocodazole (Noc), with or without 50 μg/ml Zeocin for 80 min. Total protein extract was subjected to Western blot probed with anti-H2A-S129-phospho antibody95 and anti-actin antibody (My BioSource, BSS9231831) or anti-tubulin antibody (Abcam, ab6161). Histone H2A-S129 is the target of checkpoint kinases Tel1 and Mec1. d Yeast genomic DNA from WT cells treated as in c) was analyzed by CHEF gel electrophoresis as in b) and was stained with HDGreen. Gel conditions and quantitation as b. e Exponentially growing WT cells (GA-1981) were treated with Zeocin (50 μg/ml), CMB (0.5 μM), LatA (20 μM), or with indicated combinations for 45 or 80 min. Genomic DNA was subjected to CHEF gel analysis and quantitation as in b. In bold are relevant lanes discussed in the text. f Exponentially growing YPK1 ypk2Δ (GA-5892) and ypk1-as ypk2Δ (GA-5893) cells were treated with Zeocin (50 μg/ml), LatA (20 μM), or the combination, all in the presence of 0.5 μM 1NM-PP1, which inactivates the analog sensitive (as) allele of Ypk1. Incubation time is as indicated. Genomic DNA was analyzed and quantified as in b. g The scans of signal intensities of relevant lanes (indicated in bold) are plotted for visualization but are not those used for quantitation. To the left, the scans relate lanes with bold B/A values in e and to the right, f).
To estimate the frequency of DSBs induced under our standard conditions, we modeled the number of random breaks necessary to reduce all yeast chromosomes (which range in size from 0.23 to 2 Mb) to fragments ranging from 50 to 300 kb, with an average length size around 100 ± 20 kbp35. This average fragment size theoretically requires between 80 and 140 randomly distributed DSBs35. It is estimated that Zeocin induces ss lesions in a roughly 10:1 ratio46, thus the amount of Zeocin used could induce as many as 1400 single base lesions, a level that is normally repaired rapidly in yeast by base- and nucleotide-excision repair pathways. To be able to compare rates of DSB formation under varying experimental conditions, we scanned the intensity of fluorescently stained dsDNA after PFGE and divided the signal into intensities above 570 kb (A) and below 560 kb (B, see Methods). From this, we calculate a B/A ratio that robustly quantifies the relative integrity of the yeast genome: an intact genome yields a B/A ratio <0.5, while B/A values ranging from 3 to 50 can be scored after fragmentation (Methods35; for gel quantitation and B/A ratio calculation see Supplementary Data 1).
Haploinsufficiency profiling47 showed that CMB, like BHS, impairs the growth of diploid yeast lacking one copy of various TORC1 and TORC2 subunits, and especially loss of the Tor2 kinase subunit34,35. Here we confirm that Tor2 kinase is the YCS-relevant target of CMB by challenging yeast bearing a point mutation in its conserved imidazoquinoline binding pocket34, tor2-V2126G, with Zeocin and CMB (Fig. 1b). Yeast that express only the tor2-V2126G allele were largely resistant to CMB-induced YCS on Zeocin (Fig. 1b; B/A value = 1.6 to 2.0 vs 11 to 12 in TOR2+ cells). The specificity of CMB, like BHS, is underscored by showing that the related yeast PI3 kinases Mec1 and Tel1 (ATR and ATM homologs) remained functional on CMB and Zeocin, as monitored by phosphorylation of H2A-S129 (Fig. 1c). Importantly, neither low-dose Zeocin (50 μg/ml), nor up to 10 \({{{\rm{\mu }}}}\)M of CMB, led to DSB accumulation on their own, confirming that TORC2 inhibition and Zeocin act synergistically34.
Ypk1/Ypk2 kinase inhibition and LatA trigger YCS on Zeocin
The conversion of Zeocin-induced lesions into DSBs was tested with a number of other inhibitors besides CMB. A similar but less potent fragmentation was observed with the actin polymerization inhibitor LatA, but not with nocodazole, which impairs microtubule polymerization (Fig. 1d, e). Earlier work showed that the inhibition of RNA pol II did not provoke YCS34, whereas the depletion of Ypk1/Ypk2 kinases did (ypk2Δ ypk1-as plus 1NM-PP1, Fig. 1f)34, although again less efficiently than TORC2 inhibition. To see if TORC2 and Ypk1/Ypk2 act on the same pathway as LatA, we checked the impact of combining inhibitors. On a fixed concentration of Zeocin, CMB showed partial additivity with LatA (B/A increases from 2.7 to 6.1 for CMB vs. CMB + LatA at 45 min; Fig. 1e, and gel traces, Fig. 1g), while Ypk1/Ypk2 ablation with LatA were less additive (B/A = 3.0 vs 4.8, for ypk− vs ypk− + LatA at 90 min; Fig. 1f, g). This partial epistasis suggests that the inhibition of TOR complexes by CMB does more than interfere with actin polymerization, yet all three triggers of YCS (ypk−, LatA, and TOR inhibition) do have a negative impact on actin filaments.
Synergy between actin depolymerization and Zeocin in human cells
Given that both TORC2 complex function48 and BER pathways are conserved from yeast to man49, we asked whether the synergy between Zeocin and either TOR kinase inhibition or actin filament disassembly in yeast is observed in human cells. We treated diploid primary Normal Human Dermal Fibroblasts (HDFn) for 1 h either with Zeocin alone, Latrunculin B (LatB) alone, or the two sequentially and monitored damage-induction through the intensity of H2AX phosphorylation (γH2AX)50. Note that LatB acts in a manner similar to LatA51, but is more efficient than LatA in human cells. Zeocin alone produced a low γH2AX signal indicating a DNA damage response, which was enhanced by the combination of Zeocin and LatB (Fig. 2a). To see whether a combined incubation triggers cell death, as it does in yeast, we exposed HDFn cells for one hour to titrations of Zeocin, LatB, or the two combined, and monitored cell killing by Trypan blue staining and a knockoff assay (see Methods). Neither compound alone triggered cell death after 1 h, even at the highest concentration tested, whereas Zeocin combined with 300 nM LatB left ~12% of the cells dead or dying (Fig. 2b). Thus, we detected a synergistic effect of actin filament disassembly and Zeocin-induced oxidation in primary human fibroblasts. The effect is less pronounced than in yeast, which shows up to 1000-fold higher lethality when Zeocin is combined with TOR inhibition34,35.

a Experimental flow. b Primary human dermal fibroblasts (HDFn; Life Technologies) were incubated 1 h ± 50 μg/ml Zeocin, washed, then cultured 1 h ± 300 nM LatB. Total protein extracts were probed for α-γH2AX (Merck-Milipore JBW301) and α-tubulin (Abcam ab6161) by western blot. Full blots of duplicate experiments are in Source data. b Synergistic lethality from Zeocin and LatB. HDFn cells (60,000 per sample) were incubated 1 h with indicated titrations of Zeocin, LatB, or in Zeocin +300 nM LatB. Dead cells (Trypan blue+) and suspended cells (Trypan blue-) were scored by BIO-TC20 (BioRad). c HDFn cultures were supplemented with SiR-actin (2 μM) to visualize actin, and treated 1 h with LatB (300 nM), Zeocin (60 μg/ml), or both. Fixed cells immunostained for γH2AX (green, see a), F-actin (SiR-actin, red), and DAPI (blue) were imaged and γH2AX intensity (arbitrary units) was measured by ImageJ for control cells (n = 294), LatB (n = 252), Zeocin (n = 299), and both (n = 323 cells). Arrows indicate mean intensities. Bar = 25 μm. d Alkaline Comet assay monitors ss and ds breaks in HDFn cells after 4 h on LatB (300 nM), Zeocin (60 μg/ml), or both. Comet tail moments calculated by CASP software98 for ≥200 cells each condition and plotted. Significance was determined by Mann–Whitney rank sum test. Data presented are mean value ±SEM, cells outside 95% confidence. ns = p > 0.05. Raw images uploaded as Source data file. e Inhibition of mTORC1/2 or actin depolymerization reduces HCT116 cell growth synergistically with Zeocin. Human HCT116 cells seeded at 2700 cells/well in 96-well format were treated with titration of Zeocin (x axis) and AZD8055 (mTOR catalytic inhibitor), Cytochalasin D (blocks actin polymerization), or Nocodazole (depolymerizes microtubules; y axes). Proliferation was measured after 96 h using cell titer blue (compound exposure 72 h). Scheme illustrates the isobologram with the red line showing additivity, synergism in lower gray triangle, antagonism in tan, as calculated by a combined chemical genetics method53. Blue line = 50% inhibition as f. f The corresponding average values of growth inhibition (upper panels) and synergy over model for three replicates. Colors = % inhibition; for synergy calculation see ref. 53.
We confirmed by visual inspection that the treatments had the expected impact on both DNA integrity and on filamentous actin, and quantified the γH2AX damage signal and SiR-actin structure (Fig. 2c). Exposure to either 300 nM LatB, 60 μg/ml Zeocin or both for 1 h, yielded low levels of γH2AX signal on LatB or Zeocin, which increased by 50% after the combined treatment (average values are 12.3 vs 8.1 γH2AX signal in arbitrary units; Fig. 2c). The actin cytoskeleton showed the expected disassembly in the presence of LatB, and under none of these conditions did we detect a nuclear F-actin signal. The additive effects of Zeocin and LatB on the DNA damage checkpoint kinase (γH2AX readout, Fig. 2c) is consistent with an increase of both ss nicks and DSBs, as measured by the DNA tail in an alkaline Comet assay (Fig. 2d; >50% increase in Zeocin + LatB conditions).
Given that changes in the actin cytoskeleton help drive oncogenesis6,7,8, we next tested whether actin depolymerization shows more synergy with Zeocin in a human cancer cell line, as opposed to the normal diploid fibroblasts used above. We chose a well-characterized human colorectal cancer cell line, HCT116, and tested serial dilutions of Zeocin vs either AZD8055, an mTOR catalytic inhibitor52, cytochalasin D, which inhibits actin polymerization, or nocodazole, each used at sublethal concentrations53. We did not use the CMB or BHS compounds because in mammalian cells, unlike yeast, both inhibitors target a broad range of PIKK, and not only TOR. In this synergy monitoring assay, there was a clear drop in cell proliferation when Zeocin was combined with the TORC1/2 kinase inhibitor (AZD8055) or cytochalasin D, but not with nocodazole (Fig. 2e; see synergy values from cross titrations in Fig. 2f). We hypothesize that the loss of the mismatch repair protein hMLH1 may enhance Zeocin sensitivity in HCT116 cells54 over that observed in HDFn cells, where repair pathway redundancy and an efficient short-patch BER pathway mediated by PARP, XRCC1 and Ligase III likely attenuate the synergy. We note that the SP-BER efficiency factors (PARP, XRCC1, and Lig III) are absent in yeast49,55. In conclusion, the combination of Zeocin and actin depolymerization produced a small synthetic increase in DNA damage and cell death in HDFn cells, and a pronounced synergy in cancer-derived HCT116 cells, although we do not observe the dramatic fragmentation of the genome in human cells as we do in yeast. Elsewhere we have shown that the destabilization of actin filaments by LatB or cytochalasin B in either HeLa or U2OS cells decreases SP-BER factor recruitment (XRCC1) to laser-induced damage, while inhibition of tubulin polymerization by nocodazole increased it56, which intriguingly correlates with the changes in lethality observed in HCT116 (Fig. 2e, f).
Cytoskeletal control proteins show altered phosphorylation during YCS
To understand what links the TORC2 pathway to DNA repair, we performed phosphoproteomics on yeast cells that either do or do not have Ypk1/Ypk2 activity, in the presence and absence of Zeocin, looking for changes that correlate with YCS (Fig. 3a). It was thought that identifying altered phosphorylation targets might shed light on the activities relevant for YCS, especially given the broad range of activities under Ypk1/Ypk2 control (actin dynamics, sphingolipid synthesis, and phosphotidylcholine turnover). Treatments were carried out as biological triplicates, and phosphotargets were quantified by a label-free method57,58. Among a total of 3775 detected phosphopeptides (listed in Supplementary Data 2), we considered those with ≥2-fold change over the DMSO controls and categorized these as responding either to low Zeocin alone, to the loss of YPK kinases alone, or to the combination (YCS conditions; Fig. 3b). To avoid phosphorylation events characteristic of the DNA damage checkpoint alone, we also scored the phosphoproteome in cells exposed to high levels of Zeocin (750 µg/ml), and compared these with YCS-specific hits. YCS-associated changes accounted for 656 up- and 305 downregulated phosphosites (YPKoff + Zeocin; Fig. 3b), while blocking YPK activity or adding high Zeocin affected fewer phosphosites (546 up/152 down; and 322 up/103 down, respectively; Fig. 3b). The changes characteristic of YCS identified 302 proteins containing the 389 altered phosphopeptides (Fig. 3b). Not surprisingly, phosphorylation sites on low-dose Zeocin overlapped largely with those detected at high dose Zeocin (Supplemental Data 2). The GO-term most significantly represented among the 302 YCS-specific phosphorylation targets was cytoskeleton organization (p = 2.1 × 10−11; Fig. 3c). Although no previous study had tested YCS-specific conditions, the changes we saw upon YPK inhibition are consistent with earlier work59 and corroborated a previous study that used BHS345 to identify regulators of actin organization under DNA nondamaging conditions37 (Supplementary Table 1).

a Exponentially growing ypk1-as ypk2Δ cells (GA-5893) were treated with 1% DMSO (mock), 75 μg/ml Zeocin (Ypk1on+Zeo), 0.5 μM 1NM-PP1 (Ypk1off), or both for 80 min, and genomic DNA was subjected to CHEF gel analysis as in Fig. 1b. b Treatments as in a, as well as wild-type yeast in 750 μg/ml Zeocin (high Zeo), were for 80 min, performed in triplicate. Extracted proteins were analyzed by label-free mass spectrometry (see Methods58). Phosphopeptides were triaged for change >2-fold vs DMSO control. Venn diagrams show phopshopeptides up (reddish) or down (green tones) in Ypk1off + Zeo vs. Ypk1off or Ypk1on + Zeo. Ypk1off + Zeo-specific targets are darker in the Venn diagrams. From this subgroup we subtracted phosphopeptides altered by Zeocin alone (80 up; 27 down), leaving 201 up- and 188 downregulated phosphopeptides, in 302 proteins. c GO-term analysis of the Ypk+Zeocin-specific phosphoproteome was carried out, and significance was determined vs a total number of factors in yeast in that category using the hypergeometric distribution. Cytoskeletal organization was most significantly enriched. d A cluster of interacting regulators of yeast endocytosis and actin cytoskeleton (image based on64), of which all except two (blue) were recovered as Ypk+Zeocin-sensitive phosphoacceptors. Red-labeled proteins gain phosphorylation, green lose it. e Each bar represents a specific phosphotarget site involved in actin filament regulation and endo- and exocytosis, plotted as log2 change of increased (reddish) or decreased (green) phosphorylation by indicated treatments vs DMSO. Individual values are from biological triplicates; tops of bars are median values. Relevant target sites are: Akl1: S541, S496, S504; Prk1, S553, S556, S560; Las17, T380; Pan1, S1003; S1253 or T1256; S991. T993, T995; Sla1, S449; Abp1, T206; T211; Ent1, T160, T163; T395, T388; Ent2, T468, T470, T479; Ark1. S478; Sla2, T468. T470, T479; Bni1, S325, S327, some defining overlapping phosphopeptides. Arrows indicate proteins studied further.
Among the YCS-specific cytoskeleton-related phosphotargets we found that changes were concentrated in a set of proteins that control endocytosis and cell membrane growth through actin-dependent endo- and exocytosis36,60 (Fig. 3d, e; Supplementary Table 1). The targets included Pan1 (EPS15-like), Sla1, Las17 (WASP), Abp1, Arp2, Sla2 and the yeast Epsins, Ent1 and Ent2, as well as two subunits of a regulatory kinase, Prk1 and Ark1 (Fig. 3e). Las17 is critical for the assembly of actin cortical patches61, and a complex of Pan1/End3/Sla1 co-localizes with Las17 in actin patches to regulate Arp2/Arp3-dependent actin polymerization in branched structures38,62. All of these were identified as undergoing YCS-selective changes in phosphorylation. Sla1, Las17, actin and clathrin create a complex called SLAC63, that is inhibited by the Prk1/Ark1 and Akl1 kinases36,64 which also showed YCS-associated changes (Fig. 3d). Among these targets, Las17 is recognized as an actin chaperone that can bind G-actin with multiple domains (WH1, WH2 and a polyproline-arginine enriched domain)39,65. Interaction of the Las17 WH2 domain with actin is blocked by phosphorylation on S554, which reduces F-actin at endocytic sites66, and the dephosphorylated form of Las17 contributes to the Arp2/3-mediated formation of cortical actin patches61.
Intriguingly, the most abundant phosphopeptide detected in Las17 both under YCS conditions and high Zeocin alone contained T380, and not the previously characterized phosphoacceptor S55466. The T380 residue is located between two arginine-proline stretches that are thought to allow Las17 to bind and buffer G-actin39,66 although it is unknown whether the T380-containing linker region controls actin binding. In conclusion, YCS conditions induced phosphorylation state changes that inactivated a set of factors whose physiological role is to stimulate endocytosis through actin filament growth at the plasma membrane60,64, yet it remained unclear how cortical actin regulation might interfere with BER to generate DSBs.
Depletion of Pan1 or Las17, but not of related phosphotargets, induces YCS
Given evidence in mammalian cells that WASP (the Las17 homolog) and ARP2/ARP3 moonlight in DSB repair18,22,25 and that WASP seems to regulate the RPA-ssDNA filament formation at stalled replication forks5, we decided to test whether Las17, or one of its cofactors, regulates BER in yeast. We note that yeast Pan1 has been reported to shift to the yeast nucleus in yeast under conditions of oleate toxicity67, while Las17 has not been reported in the yeast nuclear compartment and lacks the nuclear localization signal found in WASP68. We tested as well three other non-essential components of the cortical actin regulatory pathway, Sla1, Abp1, and the formin homolog, Bmi1, for roles in YCS. Our hypothesis was that the loss of one of these proteins might mimic TORC2 inhibition or possibly be resistant to it during BER-induced chromosome fragmentation.
Because both LAS17 and PAN1 are essential for yeast viability, we created strains with degron-tagged but functional genomic versions of LAS17 and PAN1. The Las17-AID and Pan1-AID fusion proteins, the only forms expressed in the resulting strains, were rapidly degraded upon addition of the plant auxin IAA (Supplementary Fig. 1a)69, yet neither the depletion of Las17 nor of Pan1 triggered DNA damage or genomic instability on its own (Fig. 4a, b, lanes +IAA, −Zeo; Supplementary Fig. 1b, +IAA), suggesting that neither factor is essential for BER or DSB repair. However, when combined with low concentrations of Zeocin, the loss of Las17 triggered massive chromosome fragmentation, similar to TORC2 inhibition (Fig. 4a; lanes +IAA, +Zeo). The loss of Pan1 also promoted YCS, albeit less efficiently (compare Fig. 4a, b; B/A = 16 for Las17 ablation, vs 1.9 for Pan1; Supplementary Fig. 1b, +IAA, −LatA), although we note that Las17-AID was more efficiently degraded than Pan1 (Supplementary Fig. 1a). Whereas Pan1 loss was additive with BHS (Fig. 4A; B/A = 1.9 increased to 17), loss of Las17 only increased slightly on BHS, as it was already highly efficient (Fig. 4b). The fact that Las17 and Pan1 ablation mimic TORC2 inhibition on Zeocin, argues that these may be the YCS-relevant targets of the TORC2-Ypk pathway, somehow altering BER regulation35.

a Exponentially growing isogenic WT (GA-5731) and PAN1-AID (GA-6810) cells were treated ± 0.5 mM IAA for 4 h to deplete Pan1. Cells were then treated with Zeocin (75 μg/ml) alone or in combination with BHS (10 μM) for 70 min. YCS was monitored as in Fig. 1b and quantitation is described in Methods, with B/A values determined as in Fig. 1 (bold values discussed in text). b As a but for isogenic WT (GA-5731) and LAS17-AID (GA-6839) cells. 0.5 mM IAA was added for 3.5 h and cells were then treated with Zeocin (75 μg/ml) ± BHS (10 μM) for 70 min. YCS was monitored, quantified and scanned as in a. Depletion of Las17 elicits efficient YCS in combination with Zeocin but not alone. c Isogenic WT (GA-5731) and LAS17-AID (GA-6839) strains were exponentially cultured and treated for 4 h with 0.5 mM IAA for 3.5 h to trigger Las17-AID degradation as in b. Cells were treated with LatA (20 μM), Zeocin (50 μg/ml), or the combination in the presence of IAA for 50 or 90 min as indicated. YCS was monitored and quantified as in a. d Isogenic WT, GA-4732 (BY4741 WT), GA-10701 (cap2Δ), GA-10905 cap2Δ las17Δ), and GA-9204 (LAS17-AID, TIR1) were cultured in SC overnight, cells were diluted to OD600 0.16 in 20 ml SC and cultured for 3 h with 0.5 mM IAA for 2 h to deplete Las17. Then equal aliquots were treated with 0.25% DMSO (control), 100 µg/ml Zeocin, 1 µM CMB, or the combination of both for 90 min. YCS was monitored, quantified and scanned as in a, with SYBR safe staining. The strain background is S288C, not W303, and therefore slightly higher concentrations of reagents were used. The cap2Δ mutation partially suppresses YCS provoked by Las17 degradation or by TORC2 inhibition.
We deleted other genes involved in actin dynamics, namely those encoding Sla1, Abp1, or Bni1 (formin), which are all targets of Akl1/Ark1/Prk1 kinases64. None provoked strong YCS on low-level Zeocin (50 µg/ml; Supplementary Fig. 1c, d). The impact of sla1 deletion was reminiscent of degradation of its binding partner Pan1 in that chromosome fragmentation was increased, yet it only had a pronounced effect at high Zeocin concentrations (200 µg/ml; Supplementary Fig. 1d). In contrast, the ablation of Abp1, a protein that specifically activates Arp2/Arp3, showed slight resistance to fragmentation in the absence BHS (Supplementary Fig. 1d), and the loss of formin paralleled wild-type at all concentrations of Zeocin tested (cf. WT vs bniΔ, Supplementary Fig. 1d). In conclusion, among various F-actin regulatory factors targeted by Ypk1/Ypk2, only the loss of Las17, and to a lesser extent Pan1, bypassed TORC2 activity and triggered YCS on low-level Zeocin.
We next asked if Las17 or Pan1 degradation were on the same pathway as LatA, by monitoring fragmentation in the absence of Las17 or Pan1 with or without LatA. Whereas the shattering triggered by partial loss of Pan1 was additive with LatA (B/A, 2.7 vs 11 and 3.6 vs 25, Supplementary Fig. 1b; +IAA, +LatA), Las17 degradation and LatA were essentially epistatic, particularly at 90 min (B/A = 9.0 vs 9.8; Fig. 4c). Given Las17’s role as a G-actin chaperone39,66, this result suggests that altered actin pools, or some other common function of LatA and Las17, drives YCS.
Further refinement of this hypothesis came from the analysis of a genetic suppressor of las17Δ. It is generally accepted that las17Δ is lethal in yeast, yet the Ayscough laboratory reported a viable las17Δ strain (KAY47339). Assuming that this strain potentially carried a suppressor mutation, we analyzed KAY 473 by genetic crossing, tetrad analysis, and genome sequencing. We confirmed the presence of an inactivating mutation in a gene called CAP2 that suppresses the lethality of las17Δ (Supplementary Fig. 1e). Interestingly, Cap2 is a non-essential factor that antagonizes actin polymerization70. To see if deletion of cap2 suppresses YCS upon the loss of las17, we created a full-length cap2 deletion, coupled it with las17Δ, and tested the double mutant for chromosome shattering provoked by low-level Zeocin, both with and without TORC2 inhibition (+CMB). The impact of cap2Δ alone was minor (Fig. 4d, B/A = 1.8 vs 2.2 upon TORC2 inhibition), but cap2Δ showed a consistent and stronger suppression of YCS triggered by loss of Las17 on Zeocin (Fig. 4d, B/A = 1.6 vs. 3.5 – CMB, and 3.7 vs 6.1 + CMB). Together with the observed epistasis of Las17 degradation with LatA, the suppression las17Δ induced YCS by cap2Δ suggests that the function relevant for preventing YCS on Zeocin is related to Las17’s role in actin polymerization39.
Las17 localization during the DNA damage response and YCS
The pathway by which Las17 regulates BER could either be direct (regulating actin at the site of damage) or indirect (by regulating global F-/G-actin balance primarily or exclusively in the cytoplasm). As mentioned, yeast Las17 lacks the NLS that is found in mammalian WASP, which presumably promotes WASP’s relocation to the nucleus under DNA damaging conditions5,18,22,25,26. It was, therefore, important to determine the subcellular localization of yeast Las17, particularly under DNA-damaging conditions.
We replaced the endogenous genes for LAS17 and PAN1, respectively, with genomic LAS17-GFP and PAN1-GFP fusions, and monitored their localization with high resolution deconvolved fluorescence microscopy, tracking actin filament dynamics with rhodamine-coupled phalloidin. Both supported normal growth, although LAS17-GFP was slightly more sensitivity to Zeocin (Supplementary Fig. 1f).
In yeast, two distinct actin structures can be visualized by rhodamine-phalloidin staining. The most dominant are cytoplasmic actin cables, which are bundles of relatively long filaments that contribute to vesicle movement and cell polarity. The second is cortical actin patches, plasma membrane-associated clusters of endocytic proteins that contain short filaments, actin-binding factors, and regulatory proteins (Fig. 5a–c). In untreated cells, cytoplasmic actin cables were clearly marked by phalloidin (Fig. 5a, red in DMSO, Zeo panels), while Las17-GFP labeled the filaments weakly and strongly lit up the focal patches of actin at the plasma membrane and in emerging buds (Fig. 5a, green). Pan1-GFP is also found in the cortical actin patches and weakly labeled filaments (Fig. 5b). Under the conditions tested, the nuclei (marked by DAPI) were devoid of F-actin (phalloidin) and GFP (both Las17 and Pan1).

a Yeast expressing Las17-GFP (GA-6804) was treated 1.5 h with CMB (1 μM), with Zeocin (100 μg/ml) or both. After fixation, DAPI (blue) and F-actin (Rh-phalloidin, red) were captured by spinning disk confocal microscopy. Images are maximum-intensity projections of focal stacks acquired in each channel (see Methods). Bar = 5 µm. b Yeast expressing Pan1-GFP (GA-6764) was treated and stained as in a. DAPI alone is not shown. Bar = 5 µm. c Scheme of a budding yeast cell illustrating structures of interest. d Las17-GFP expressing cells were treated as in a, but stained with 20 nM Mitotracker Red CMXRos (Thermo Scientific) and DAPI (blue). Colocalization of Las17-GFP and mitochondria is quantified in e. Bar = 5 µm. e Yeast expressing Las17-GFP or Pan1-GFP (see a, b) were split and treated 1.5 h either with DMSO (Control, blue) or 1 µM CMB and 150 µg/ml Zeocin (YCS, black). Colocalization of Las17-GFP or Pan1-GFP (green) and Mitotracker (red) was quantified by determining the Pearson correlation coefficient in each single plane of an image stack. n = 118 nuclei for Las17-YCS, all others n = 78; line = median. r values show a robust correlation of Las17 with mitochondria. Repeated twice. Quantitations in Source Data file. f Las17-GFP is not enriched in nuclei on Zeocin. Las17-GFP (green) expressing cells were treated ± 500 μg/ml Zeocin for 1 h at 30 °C, fixed and counterstained with DAPI (blue). Image stacks (green and UV) were analyzed for nuclei spanning at least 5 planes. Nuclear GFP intensity was measured per plane and averaged across ≥5 planes for mean intensity per nucleus, plotted as arbitrary GFP units. n = 300 nuclei without Zeo, mean and S.D. = 2861±700; n = 183 with Zeo, mean and S.D. = 3002±805. Unpaired two-tailed T test with Welch’s correction, p = 0.051. See Source Data Files. g Concept of DamID mapping71 of Las17- and Orc2-Dam fusions, derived from an existing sketch72. h Wild-type (GA-1981) cells expressing Dam, Las17-Dam, or Orc2-Dam were treated ±300 μg/ml Zeocin for 1 h at 30 °C. Genomic DNA digested with DpnI (cleaves at GmATC) or Sau3AI (cleaves GATC, methylation indifferent), was run on 1% agarose gels; stained with SYBR safe or rDNA by Southern blot (see Methods).
Upon TORC2 inhibition, Las17-GFP showed a striking relocation to small, bright round foci that are DAPI-positive, and are adjacent to, yet clearly distinct from, the cell nucleus (Fig. 5a, panels CMB). MitoTracker dye and quantitation of the correlation coefficient showed that these structures are mitochondria (Fig. 5d, e). On Zeocin alone, on the other hand, Las17 and Pan1 remained strongly associated with cortical actin foci and filaments near the plasma membrane (Fig. 5a, b). Under YCS conditions (CMB + Zeo), not only are cytoplasmic actin filaments lost presumably due to YPK inhibition, but Las17 is lost from cortical actin patches (Fig. 5a) and shifts to DAPI-positive mitochondrial clusters (Fig. 5d, e) distinct from the nucleus. Pan1 does not show the same relocalization (Fig. 5b, Supplementary Fig. 2a). We integrated and quantified potential Las17-GFP signal in the nucleus by using DAPI to define the nuclear sphere, yet there was little or no increase in nuclear Las17-GFP signal (Fig. 5f) in response to low or even very high dose Zeocin (500 μg/ml).
We thought it possible that fluorescence microscopy was not sensitive enough to detect a small pool of nuclear Las17 under YCS conditions. We, therefore, used a more sensitive assay for the detection of proteins associated with DNA, namely DamID71. We fused the catalytic domain of the bacterial Adenine methyl transferase (Dam) to Las17 and expressed it at a low constitutive level, which allows detection of even transient interactions of the Dam fusion protein with the genome through GmATC detection71 (see sketch, Fig. 5g, modified from ref. 72). Adenine methylation at GATC motifs is detected by restricting total genomic DNA with Dpn1, which recognizes GmATC, rendering Dpn1-cleavage a highly sensitive surrogate marker of protein-DNA interaction. As controls, we expressed the Dam methylase alone, and Dam fused to a known DNA binding protein, origin binding factor Orc2 (Fig. 5g). Cleavage by methylation-insensitive Sau3a is a positive control for GATC site frequency.
Upon the introduction of Las17-Dam in cells, we found no increase in Dpn1 cleavage either with or without Zeocin (Fig. 5h), and on the contrary, expression of the Las17-Dam fusion produced less Dpn1 cleavage than Dam alone, suggesting that Las17 sequesters the methylase away from the nucleus. Orc2-Dam and Dam alone both showed significant DNA association, and neither was influenced by Zeocin (Fig. 5h). Probing genomic DNA by Southern blot for the highly repetitive rDNA array confirmed the observations on total genomic DNA (Fig. 5h). Similar results were observed in the presence of TORC2 inhibition. These results reinforce the Las17-GFP localization results and argue against a direct role for Las17 in the nucleus, at least with respect to Zeocin-induced lesions. This suggests that Las17 acts indirectly to protect cells from misregulated BER and DSB induction35.
Does Las17 act through mitochondrial respiration or the LINC complex?
The striking colocalization of Las17 with mitochondria, as well as its presence in a small focus near the Spindle pole body (yeast centrosome) under YCS conditions (arrows, Supplementary Fig. 2b), led us to test mitochondrial function and the LINC complex in YCS. The Sun-domain protein Mps3 is a key component of LINC, which links the actin cytoskeleton to the genome through the inner nuclear membrane31,32(Supplementary Fig. 2c, d). However, neither mutation of LINC complex components Mps2 or Mps3 (Supplementary Fig. 2d), nor ablation of ATP synthesis in the mitochondria by mutating the F1F0 ATPase (atp3Δ; Supplementary Fig. 2e), altered the yeast cell sensitivity to YCS. We conclude, therefore, that active mitochondrial ATP synthesis is not required for YCS, nor is any cytoplasmic-nuclear communication mediated by LINC. Nonetheless, this does not rule out subtle roles for mitochondrial reticulation73,74, which occurs on LatB and upon Las17 ablation. We note that Apn1, which cleaves the DNA backbone at abasic sites, is found both in mitochondria and nuclei75.
Las17 degradation alters the F-/G- actin ratio in favor of G-actin
Previous work argued that the multiple binding sites for G-actin on Las17 might serve to prevent an imbalanced F-/G- actin ratio39,66, in parallel to its ability to nucleate actin filaments and stimulate Arp2/3-mediated branching. To see if this occurs under YCS conditions, we monitored the number of cells with visible actin filaments under relevant conditions, by staining with fluorescent phalloidin after fixation (see examples of images in Supplementary Fig. 3; quantified in Fig. 6a, b). Yeast was incubated in YCS conditions (CMB or degradation of Las17-AID by IAA, with or without Zeocin), and we scored the presence of actin filaments and bundles. Given that actin filaments are most visible during S phase in budding yeast, we also scored the fraction of budded cells (S phase). All scoring was performed double-blinded by three independent persons, and the combined ratios were plotted (Fig. 6a). Budding index varies slightly under the conditions scored (Fig. 6b), but this is factored out as we calculated a ratio of budded cells with actin filaments over all budded cells. As expected from imaging data, both the degradation of Las17-AID and incubation with CMB strongly reduced the fraction of cells bearing actin filaments, with or without Zeocin (Fig. 6a). To deduce whether actin was degraded or converted to G-actin, we measured overall actin levels in two ways. First, because Cofilin binds both F- and G-actin, we used a previously described, fully complementing Cof1-RFP allele76 to monitor actin levels under YCS-favoring conditions (Fig. 6c; sample images in Supplementary Fig. 3a, c). We also probed the total actin levels in a fixed number of cells by western blot, using tubulin as a loading control (Fig. 6d).

a Fraction of budded cells or total cells with F-actin filaments was determined after staining with Rh-phalloidin. Exponentially growing cells (GA-5731, AID control; GA-6840, Las17-AID; GA-1981, WT) were treated as indicated (±0.25 mM IAA for Las17-AID and its control, and 50 μg/ml Zeocin ± 0.5 μM CMB for WT), then fixed and stained with Rh-phalloidin. Image examples in Supplementary Fig. 3. Both brightfield and fluorescent images were scored for budding index and cells with filaments by three independent operators on blinded images. Number of cells scored per condition is shown in b. Plots show % budded cells with filaments (left) or % total cells with filaments (right). Data presented as mean value (top of bar) ±SEM. Raw numbers in Source Data file. b Budding index (bud presence) was scored for the same cell populations analyzed in a by three operators on blinded images. Total number cells are same for a, b. Plotting Budded cells with filament/Budded cells excludes cell cycle impact on results. c Yeast strains as a were transformed with a Cof1-RFP (internal tag, Addgene #37102) expression plasmid and treated as above. Cof1-RFP intensity monitors both G- and F-actin and was quantified for each condition with a CellProfiler pipeline. Each dot represents one cell and the number of cells scored range from 402 to 1731, as indicated within each bar. All data points are plotted and top of bar indicates median. By Wilcoxon rank sum test actin levels do not vary significantly ± IAA nor under YCS conditions. d Cells of the samples used in a were collected before fixation. Total protein extracts were subjected to SDS-PAGE and western blotting with validated anti-actin (My BioSource, BSS9231831) or anti-tubulin (Abcam, ab6161). e Scoring nuclear actin detected through Cof1-RFP (expressed as in c) using nuclear masking based on DAPI staining (Fig. 5f) on WT and an isogenic strain lacking Las17 (LAS17-AID + IAA). Samples visualized as c. Bar = 10 μm. In graph of mean (bar = mean values) of n nuclei (n = 19, WT; Mean = 0.8339 ± 0.0131; n = 20 Las17-AID; Mean = 0.1316 ± 0.0340). Based on a two-tailed T test p < 0.0001. Data in Data Source file.
In contrast to actin filaments, total actin levels do not decrease upon Las17 degradation based on Cof1-RFP signal, nor in wild-type cells incubated in CMB, either with or without Zeocin (Fig. 6c, d). Given that actin filaments were reduced 3- to 5-fold (Fig. 6a), but overall actin signal does not change, we conclude that G-actin levels increase as actin filaments disappear. In other words, Las17 degradation and/or exposure to CMB increases the balance of G-actin over F-actin.
To determine whether G-actin remained cytoplasmic or became enriched in the nucleus as Las17-AID was degraded, we monitored single confocal planes of Cof1-RFP signal, and integrated this signal within a nuclear mask, generated from the DAPI staining (Fig. 6e, sample images at left). Quantitation showed a significant increase in nuclear actin upon Las17 degradation (Fig. 6e, p < 0.001). This points to an important role of Las17 in regulating the G- and F-actin balance, consistent with evidence that its polyproline-arginine rich domains bind multiple G-actin molecules weakly, while its WH2 domain provides high-affinity G-actin binding39,66. If we truncate Las17 at aa 295, just upstream of this polyproline domain, the residual protein should still interact with Arp2/Arp3 but fail to buffer actin39. Consistent with the notion that G-actin binding by Las17 protects wild-type cells from YCS on Zeocin, the las17(1–295) allele rendered cells highly sensitive to low levels of Zeocin (Supplementary Fig. 1f).
The act1-111 allele leads to CMB-insensitive Zeocin-induced breaks
We reasoned that if actin is the culprit that triggers YCS on low-dose Zeocin, then artificially increasing nuclear actin might also provoke YCS. This is indeed the case, as shown in Shimada et al.35, the ectopic expression of actin mutants lacking the nuclear export signal (act1nes) provoked weak but detectable chromosome fragmentation, even without TORC2 inhibition (B/A ratios 1.4 to 2.3; see ref. 35). This was shown not only for wild-type actinnes, but for actin forms that either favor or impair filament formation (act1-S14C and act1-A204E/P243K, respectively), as well as one filament-forming mutant that is impaired in lateral protein-protein interactions (act1-11177,78). This non-lethal actin mutation, act1-111, has an altered acidic patch on the lateral side of the molecule (Fig. 7a; point mutations D222A, E224A, and E226A78), which slows down endocytosis and cell division due to the partial dissociation of actin cables79 and an inability to bind the Las17 polyproline domain39. Nonetheless, act1-111 protein remains at wild-type levels (Fig. 7b, c) and the act1-111 allele was hypersensitive to Zeocin (at 20 μg/ml, Fig. 7d), reminiscent of the Zeocin sensitivity observed for the Las17 truncation that eliminates its G-actin-binding domain (Supplementary Fig. 1f). Importantly, act1-111 sensitivity to Zeocin was fully complemented by introduction of a single copy wild-type pACT1 plasmid (Fig. 7d).

a Position of act1-111 (red) and act1-129 (green) mutations77 on an actin monomer shown in two orientations. Filament formation is intact through pointed and barbed ends. b Actin cytoskeleton staining in act1-111 (GA-8592) ±pACT1. Exponentially growing cells were fixed with PFA, stained Rh-phalloidin for 2 h, and imaged. The phalloidin signal in act1-111 expressing pACT1 resembles wild-type, while act1-111 has mostly cortical F-actin patches. Quantitation of actin cables was scored by two blinded operators on three isolates of the act1-111 mutant vs act1-111 + pACT1. n total +pACT1 = 1012, n total act1-111 = 2790; data are presented as mean value ±SEM, by two-tailed unpaired T test, p < 0.0001. Scoring is in Source Data file. Scale bar = 10 μm. c Actin levels are constant under YCS conditions in act1-111 strains. Whole protein extracts from strains grown as in b were analyzed by western for a nuclear pore protein (Mab414, Abcam) and actin (My BioSource). Quantitation reflects four blots scanned by the Typhoon scanner; Actin:Mab414 value on YCS is normalized to +pACT1. d The act1-111 mutant (a) is hypersensitive to Zeocin. 1:10 dilution series of act1-111 ± pACT1 on SC agar plus glucose with indicated Zeocin concentrations. Colonies grew 3 days at 30 °C; performed in triplicate with similar results. e Las17-GFP act1-111 cells (GA-8592) ± pACT1 plasmid are resistant to YCS. An isogenic wild-type background (GA-9247; BY background) and the act1-111 mutant were treated with 2 µM CMB and 150 µg/ml Zeocin for 1.5 h, and genomic DNA was monitored by CHEF gel analysis. Where indicated, 50 µM LatA was added rather than CMB. Gels and quantitation are as Fig. 1b. B/A values show that act1-111 is resistant to CMB and LatA treatment. Experimental repeats (n = 3) include panel f. f Wild-type and act1-111 (GA-8592) and act1-129 cells (GA-8590) ± pACT1 plasmid were treated 1.5 h with Zeocin (125 µg/ml), a titration of CMB (0.25–2.5 μM) or Zeocin and CMB as indicated. Genomic DNA was monitored by CHEF gel, stained, and quantified as Fig. 1b. The act1-111, but not act1-129 cells show less CMB-induced fragmentation. The experiment was repeated three times.
To see if act1-111 affects YCS, the mutant strain expressing act1-111, either complemented or not by +pACT1, was screened for chromosome fragmentation on Zeocin, in the absence and presence of CMB (Fig. 7e, f). The act1-111 protein, rather than enhancing YCS as it did when accumulated in the nucleus due to mutated export signals35, suppressed fragmentation in the presence of either CMB or LatA (Fig. 7e, f; bold values; B/A = 0.87 vs 8.1 for wild-type on CMB). This resistance was overcome by expressing wild-type ACT1 (+pACT1, Fig. 7e, f). In contrast, another non-lethal actin mutant, act1-129 (R177A, D179A; green in Fig. 7a)77, which allows actin assembly into cables, patches, and microfilaments, but fails to bind certain proteins such as Abp1, was only weakly compromised for YCS (Fig. 7f; B/A = 7.2 or 6.5 vs 2.5 for act1-111).
The act1-111 mutation does not alter Las17 distribution under YCS conditions (i.e. CMB + Zeocin, Supplementary Fig. 4), allowing Las17 association with mitochondria and no visible enrichment of Las17 in nuclei, resembling wild-type cells. Since Las17-GFP co-localizes with mitochondria even under conditions that suppress chromosome fragmentation (cf. act1-111 vs. act1-111 + pACT1; Supplementary Fig. 4), we can conclude that the shift of Las17 to mitochondria is not sufficient to trigger YCS.
The actin-containing chromatin remodeler INO80C is required for YCS
Besides interfering in Las17 binding, the residues mutated in act1-111 (D222A, E224A, and E226A) are found at the binding interface of actin and Arp8, an integral component of the multisubunit INO80C remodeler (Supplementary Fig. 580,81). The mutations destabilize an important alpha helix that forms salt bridges to Arp8. Whereas G-actin forms a heterodimeric complex with Arp4 in chromatin multiple modifying complexes (notably, INO80C, SWR-C, and NuA412,14,44; Fig. 8a), its interaction with the INO80C-specific subunit Arp8, creates an Arp8-Arp4-actin-Ies4 module that binds the HSA domain of the Ino80 catalytic subunit (aa 356–69181;). This subcomplex plays a central role in the ability of INO80C to bind its substrate nucleosome during remodeling reactions13,80,81. Knowing that INO80C remodeling activity promotes the processivity of DNA replication polymerases, both in recovery from fork stalling40,41,43 and in an unchallenged S phase45, we next asked whether the changes in G-/F- actin ratio provoked by Las17 degradation and TORC2 inhibition, influences long-patch BER through nucleosome remodeler activity35. We note that the study of nucleosome remodeler mutants, and especially ino80 mutants in yeast, requires the study of more than one background, as ino80Δ is lethal in W303-derived strains, but viable in the S288C background44,82. Moreover, the two backgrounds have different sensitivities to some DNA-damaging agents.

a Sketches of subunit composition of the S. cerevisiae INO80C, SWR-C and NuA4 chromatin remodelers and modifiers each containing actin as a core subunit12,13,14,15. Subunit names are from budding yeast. b Individual ISWI and CHD remodelers are not needed for YCS, while loss of Arp8, a unique INO80C subunit confers partial resistance. Exponential cultures of wild-type (WT) W303 (GA-9875); isw1Δ,isw2Δ (GA-9878); chd1Δ (GA-9879); isw1Δ isw2Δ chd1Δ (GA-9882) and arp8Δ (GA-9848) were treated for 1.5 h with 0.5 μM CMB and 50 μg/ml Zeocin (+) or 1 μM CMB and 100 μg/ml Zeocin (++), prior to processing for CHEF gel analysis. Quantitation as in Fig. 1b. c Isogenic strains in the BY background lacking indicated remodeler subunits of SWR-C or NuA4 were tested in the YCS assay. Note that this background is more resistant to Zeocin than W303. BY4733 (GA-2263), bdf1Δ (GA-9953), arp6Δ (GA-2319), eaf1Δ (GA-9954), eaf7Δ (GA-9955) were grown to exponential phase and treated for 1.5 h with DMSO (−), 0.5 µM CMB and 80 µg/ml Zeocin (+) or 1 µM CMB and 125 µg/ml Zeocin (++), prior to CHEF gel analysis. Quantitation as in Fig. 1b. The eaf7Δ mutant showed slight resistance at low concentrations of Zeocin/CMB, while arp6Δ and eaf1Δ mutants showed minor resistance at the higher concentration. Except for INO80C, the minor effects were inconsistent for a given remodeler. d Deletion of arp8 delays YCS consistent with a requirement for functional INO80C for polymerase processivity during LP-BER. The indicated isogenic strains (ARP8 + = GA-9247; arp8Δ = GA-9250) were incubated with the indicated compounds for either 45 or 90 min, prior to processing for CHEF gel analysis. Quantitation is as in Fig. 1b. At 45 min ARP8+ started to show fragmentation, unlike arp8Δ. The experiment was repeated twice. e The ATPase mutant in the catalytic subunit ino80 (K737A) confers resistance to YCS. Performed in the BY background, ino80Δ (GA-2264) cells bearing Cen-plasmids expressing WT INO80 or ino80 K737A were treated for 1.5 h with 0.5 μM CMB and 80 μg/ml Zeocin (+) or 1 μM CMB and 125 μg/ml Zeocin (++); CHEF gel analysis and quantitation as Fig. 1b. Experiment repeated twice.
We performed the YCS assay on mutants of core subunits of the INO80, ISW1/ISW2, SWR-C, NuA4, and CHD1 remodelers, under two different concentrations of Zeocin and TORC2 inhibitor and, where necessary, in both the W303 and S288C (BY) backgrounds. We tested the actin-independent Isw1 and Isw2 remodelers because they have been proposed to act on DNA replication polymerase processivity in a chromatin context42,43,83. We found that the isw1Δ isw2Δ double and the chd1Δ single mutant was actually more susceptible to YCS than the isogenic wild-type W303 strain, whereas the arp8Δ mutant, which significantly reduces INO80C activity and fork processivity40,41,43 was partially resistant to shattering (B/A = 4.14 vs 21.8, arp8Δ vs. wild-type on 50 μg/ml Zeo + CMB; Fig. 8b). This resistance was less pronounced at higher Zeocin levels, but was also observed in the S288C-derived BY background (Fig. 8d). It is not clear why the isw1Δ isw2Δ double mutant is more susceptible to YCS, but the fact that the triple isw1Δ isw2Δ chd1Δ showed weak resistance (B/A = 6.6 vs 21.8, on 50 μg/ml Zeo + CMB, Fig. 8b) may reflect altered nucleosome spacing and changes in lesion accessibility in the mutant84. The resistance we observe in arp8Δ cells is consistent with data showing that INO80C promotes DNA polymerase δ and ε processivity40,41,43, as both polymerases have been implicated in long-patch BER35. Our finding that resistance drops at higher doses of Zeocin is also consistent with this model, as the higher the density of oxidative lesions, the less impact polymerase processivity should have35.
With respect to other actin-containing remodelers, deletions of SWR-C subunits (Bdf1) or NuA4 subunits (Eaf1 or Eaf7) showed no pronounced resistance to YCS over the wild-type BY background at the lower Zeocin concentration (80 μg/ml Zeo + CMB, Fig. 8c). Importantly, despite a partial resistance to YCS observed for the arp6Δ allele (B/A = 3.3 vs 7.1, Fig. 8c), ablation of the catalytic subunit of the SWR-C complex, swr1Δ, led to enhanced YCS while loss of its ATPase activity had no effect (Supplementary Fig. 6a). Similarly, the deletion of SNF2 and SWI3, which encode subunits of the Snf2/Swi remodeler, failed to alter YCS efficiency (Supplementary Fig. 6b).
In the arp8Δ background the INO80 complex lacks a key structural module (Arp8, Arp4, actin, Ies4), and has a decreased (but not abolished) rate of remodeling7. We, therefore, asked whether YCS in arp8Δ yeast might be delayed, rather than abolished, by testing YCS after 45 min and 90 min exposure to CMB and Zeocin (Fig. 8d). Indeed, resistance to YCS conditions was more pronounced at 45 min (relative to the ARP8+ strain) than at 90 min (B/A = 0.96 vs 3.7 at 45 min; 2.2 vs 6.5 at 90 min; Fig. 8d). Finally, we tested the ATPase-dead INO80 allele, by expressing the DEAQ-box dead ino80-K737A mutant in the ino80Δ BY background. We found that the K737A ATPase mutant was resistant to YCS at high dose Zeocin (B/A = 3.1 vs 13.0 for the WT background; Fig. 8e). Together, these results suggest that the INO80C remodeler facilitates YCS under conditions that increase G-actin levels in the nucleus. Importantly, without CMB the ino80 and arp8 mutants are indistinguishable from wild-type in their response to Zeocin with respect to chromosome fragmentation (B/A = 0.78, 0.74, 0.63, 0.67; Fig. 8d).