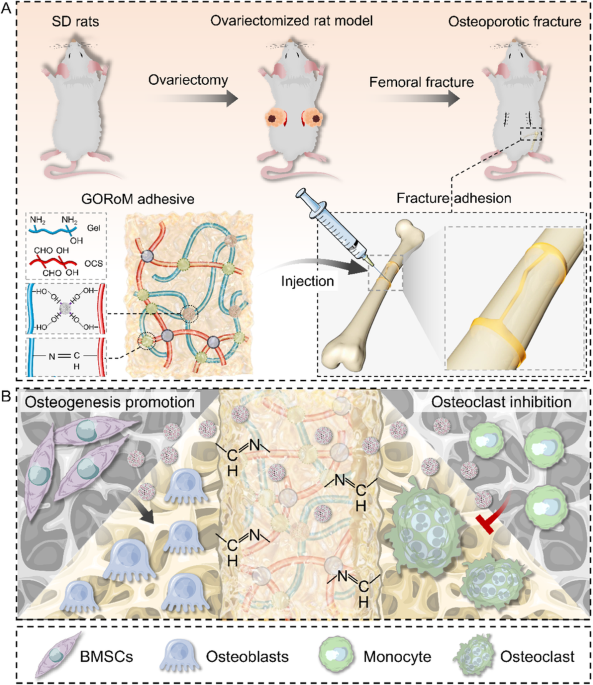
Preparation and characterization of GORoM hydrogel
For the fabrication of the GORoM hydrogel (Fig. 2A), OCS was synthesized based on a previously established protocol21. Fourier transform infrared (FTIR) analysis revealed the presence of a novel infrared band at 1725 cmâ1 in the OCS spectra (Fig. 2B), which was attributed to the stretching vibration of aldehyde groups, confirming successful OCS oxidation. Subsequently, the MBGNs were synthesized following a previously outlined protocol20. Romosozumab was then loaded onto the MBGNs to create romosozumab-loaded MBGNs (RoM). At 37â°C, gelatin (30% (w/v)) and OCS (10% (w/v)) containing RoM (5% (w/v)) were combined to form the GORoM hydrogel. The interaction between the gelatin hydrogel skeletonâs free amino group and the aldehyde group of OCS facilitated reversible covalent crosslinking through a Schiff base bond. Rheological analysis indicated that the storage modulus (Gâ) of all three adhesives exceeded the loss modulus (Gâ), confirming their stability as viscoelastic solids23. Furthermore, no difference in the storage modulus was noted between the GOM and GORoM groups (2351â±â20 vs. 2251â±â50âPa), underscoring that romosozumab did not influence the mechanical properties of the GOM hydrogel (Fig. 2C). SEM demonstrated the transparent porous structure of the hydrogel, with white MBGN particles distributed on the pore wall (Fig. 2D). This coarseness is attributed to the adherence of MBGNs, which contributes to a three-dimensional habitat that facilitates cell attachment, growth, and division. The porous structure of bioadhesive materials is integral to their design, enhancing their utility in osteoporotic fracture repair by improving their surface area, drug loading capacity, adhesive strength, and overall bone remodeling processes. Additionally, this structure has a specific effect on cellular activities. TEM highlighted the large surface area and mesoporous structure of the microspheres (Fig. 2E). The spatial distribution of romosozumab-loaded MBGNs within the hydrogel was revealed through three-dimensional immunofluorescence imaging (Fig. 2F). The biodegradability of the GORoM-gel was extensively evaluated. In vitro, the gel initially showed rapid degradation, with a significant reduction in mass within the first 14 days (Fig. S1A). In vivo observations mirrored this pattern, with a notable decrease in the gel bulk over time and minimal residue remaining by Day 14. These findings underscore the efficacious biodegradation of GORoM-gel both in vitro and in vivo (Fig. S1B). The degradation of the hydrogel can also be influenced by the concentration and release profile of bioactive substances, such as romosozumab, in the GOM hydrogel. The release of more than 80% of the loaded romosozumab over 14 days could affect the degradation rate of the hydrogel, initially causing rapid degradation both in vitro and in vivo. Collectively, these findings indicated the successful integration of romosozumab-loaded MBGNs into the GO hydrogel. Romosozumab release from the GOM hydrogel was sustained for more than 14 days, with more than 80% of the loaded romosozumab being released within this timeframe, ensuring substantial biological efficacy for fracture healing (Fig. 2G, H). Importantly, the GORoM hydrogel precursor exhibited excellent injectable properties, as it easily formed a stable gel when it was injected into water or into a petri dish using a syringe (Fig. 2I, J). The fracture repair ability of the adhesive was also demonstrated by splitting and reassembling the adhesive tablets (Fig. 2K). Finally, when applied to a gloved finger, the freshly prepared GORoM hydrogel adhered firmly to the glove surface (Fig. 2L). Collectively, these results validate the successful fabrication of the GORoM hydrogel.
A A physical image of the GORoM hydrogel (scale bar: 5âmm). B FTIR spectra of CS, OCS, MBGN, and gelatin. C Mechanical properties of GO, GOM, and GORoM. D The microstructures of the GO and GORoM hydrogels were observed via SEM. Scale bar: 20 μm and 1 μm. E TEM images (scale bar: 50 μm) of MBGNs. F Fluorescence images of labeled GORoM. Red, green, and blue indicate RhB-labeled romosozumab, FITC-labeled MBGNs, and DAPI-labeled gelatin, respectively. Scale bar: 100 μm. G The cumulative release profile of romosozumab with or without the GOM hydrogel after 14 days (nâ=â3). H The daily release curve of romosozumab with or without the GOM hydrogel (nâ=â3). I The GO-based adhesive can be smoothly injected into PBS to maintain a gel-like consistency. J The GO-based adhesive can be injected and used to write the letter âMâ. K Demonstration of the self-healing capacity of the hydrogels by cutting gelled GORoM hydrogels into halves and rejoining them to obtain healed interfaces. L The picture shows that the gelated GORoM hydrogel firmly adhered to the glove, demonstrating good adhesion. M Photographs showing that the GORoM hydrogel can be used to firmly resample freshly produced porcine-awed femur bone pieces, as evidenced by successful lifting of a 2.8âL water bottle. N, O Representative stressâdisplacement curve of the endâto-end tensile test. P, Q Representative stressâdisplacement curve of the lap-shear tensile test. R Reversible adhesiveness of the GORoM adhesive was demonstrated by rejoining and reconnecting breaking bone pieces. S Reversible adhesiveness of the GORoM adhesive was demonstrated by rotating one of the bonded bone pieces 180°.
Characterization of ex vivo adhesiveness to porcine bone
Effective tissue adhesiveness is critical for bone adhesives because it ensures swift and stable adherence to the defect site and facilitates cell recruitment for accelerated bone healing. The adhesiveness of the GO-based adhesives was assessed using fresh cancellous bone pieces from porcine femurs. The GORoM adhesive could rejoin bone fragments, as evidenced by its application to simple or severely comminuted fresh porcine femur fracture models (Fig. 2M). The reassembled freshly sawed porcine femur bone segments could even support the weight of a 2.8âL water bottle (Fig. 2M). While the GO hydrogel and GORo exhibited low adhesive strengths (58.3â±â2.5 kPa and 59.1â±â1.3 kPa for end-to-end, 40.2â±â2.2 and 44.1â±â3.6 kPa for lap-shear) before adhesive failure (Fig. 2N, O), both the GOM and GORoM hydrogels exhibited significantly greater adhesive strengths, exceeding 162â±â5.6 kPa and 164.7 kPa ± 6.3 kPa for end-to-end tests (Fig. 2N, O), as well as 205.8 kPa ± 4.2 kPa and 213.3 kPa ± 7.1 kPa for lap-shear tests (Fig. 2P, Q), highlighting their excellent adhesive capabilities. In addition, we tested the effect of adding different concentrations of MBGNs on hydrogel stiffness, and the results showed that adding 10% MBGNs was the most appropriate treatment (Fig. S2). Incorporating 10% (w/v) MBGNs significantly improved the crosslinking density through physical and covalent mechanisms, enhancing the hydrogelâs cohesion. Furthermore, Ca2+ released from the MBGNs facilitated Schiff base reactions at the adhesive-bone tissue interface, enhancing interfacial adhesion24,25. These findings demonstrated the suitability of the GOM hydrogel for assisting in bone fracture fixation.
In addition to adhesive strength, flexibility is equally crucial. Adequate flexibility and adjustable adhesion provide surgeons with ample maneuvering space for aligning fracture fragments, which often requires numerous adjustments for optimal fracture reduction26. During demonstrations, we reconnected the broken pieces, rotated one segment by 180°, and reconnected the fragments (Fig. 2R). As the GORoM adhesive self-healed at the reconnected interface, the boundary between the fragments became indistinct, resulting in instant readhesion upon reassembly. Flexibility was further exemplified by rotating a reconnected bone segment by 180° (Fig. 2S). These results underscore the rapid self-healing ability of hydrogels prior to complete crosslinking, which relies on reversible intermolecular hydrogen bonding and dynamic covalent Schiff base networks. In summary, the flexibility and adjustability of these adhesives allow surgeons ample time for effective bone fragment reattachment.
In vitro and in vivo biocompatibility
Robust biocompatibility is imperative for bone adhesives. The survival, proliferation, and adherence of the GORoM hydrogel were assessed through the seeding of BMSCs onto hydrogel surfaces. These cell behaviors are pertinent to adhesive-bone integration and subsequent osteoporotic fracture healing. Live/Dead staining conducted one day after coculturing cells with hydrogels revealed a majority of live cells with green fluorescence (>90% cell viability) and a small number of red fluorescent dead cells (Fig. 3A). After 1, 3, and 7 days of coculture, the optical density values progressively increased due to cell proliferation, with no significant differences observed among the four groups (Fig. 3B). All the cells stained with Amanita phalloides exhibited distinct pseudopodia shapes with F-actin expression after 7 days of coculture (Fig. 3C). Furthermore, no significant difference in cell morphology was noted among the groups after 7 days of coculture. These observations corroborate the strong biocompatibility of each adhesive, indicating their safety for use.
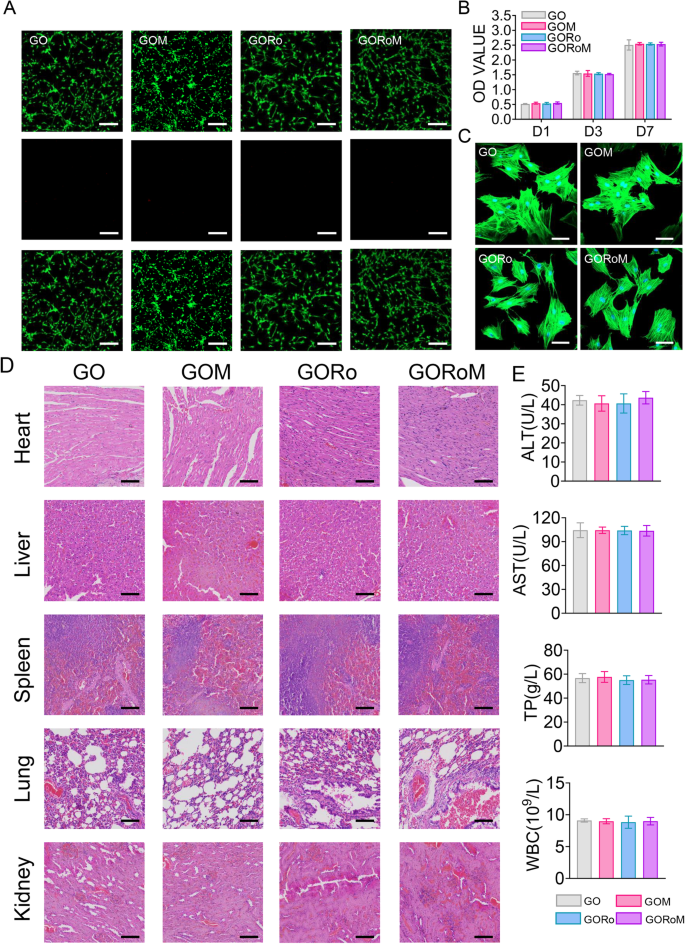
A Live/Dead staining of BMSCs after 24âh of coculture with different hydrogels. Live cells are marked with green fluorescence, and dead cells are marked with red fluorescence (scale bar: 200 μm). B CCK-8 assay results for BMSCs cultured on the hydrogels for 1, 3, and 7 days. C Cytoskeletal staining photos of cells cultured on the hydrogel after 2 days (scale bar: 50 μm). D Pathological images of the heart, liver, kidney, and lung of mice in each group (scale bar: 50 μm). E Serum levels of biomarkers reflecting liver and kidney function and blood cell parameters in the rats treated with the GORoM hydrogel (nâ=â3). *pâ<â0.05, **pâ<â0.01, and ***pâ<â0.001.
In terms of in vivo biocompatibility, hydrogels from each group were subcutaneously injected into the backs of the rats. Histopathological analysis and organ-related blood tests (heart, liver, kidney, lung, and spleen) were used to evaluate the in vivo biocompatibility of each adhesive. Notably, no significant accumulation of hydrogel degradation products was found in the main organs of the rats, and no pathological abnormalities were observed (Fig. 3D). Moreover, the hydrogel groups exhibited no significant differences in the TP, ALT, AST or WBC levels compared to those of the control group (Fig. 3E), confirming the favorable in vivo biocompatibility of the hydrogel.
Osteogenic differentiation of BMSCs on GORoM
The dysregulation of bone homeostasis during osteoporosis, characterized by decreased bone-forming osteoblast activity and increased bone-resorbing osteoclasts, underscores the importance of re-establishing this balance to enhance osteogenesis and reduce osteoclastogenesis during fracture treatment27,28. The osteogenic effects of the GORoM hydrogel were explored using BMSCs cultured on the hydrogel surface. After 7 and 14 days of osteogenic induction, alkaline phosphatase (ALP) staining, alizarin red (ARS) staining, and mRNA expression (collagen type I [Col-1], osteocalcin [OCN], runt-related transcription factor 2 [RunX2], and osteopontin [OPN]) related to osteogenesis were assessed. The enzyme ALP, found on the surfaces of osteoblasts, is a widely acknowledged indicator of bone metabolism29. ALP staining and substantial ARS staining were observed across all hydrogel surfaces, with the GORoM hydrogel demonstrating more intense staining (Fig. 4A, B) and larger mineral nodules (Fig. 4C, D) on Days 7 and 14 than did those of the GO and GORo groups. This finding highlights the coordinated promotion of osteogenic differentiation by MBGNs and romosozumab in the GORoM hydrogel. These findings were corroborated by the mRNA expression levels of OPN, RunX2, OCN and Col-1 (Fig. 4E, F). Moreover, a similar trend was observed in the protein expression levels of OPN and RunX2, indicating an osteogenic synergistic effect from additional MBGNs and romosozumab (Fig. 4GâJ). MBGN degradation releases Ca2+ and SiO44â, which regulate stem cell osteogenic differentiation and bone formation30,31. The binding of romosozumab to sclerostin, an inhibitor of the Wnt/β-catenin signaling pathway, has been shown to enhance the survival of osteoblasts and osteocytes while reducing osteoclast occurrence32. This phenomenon occurs through an increase in osteoprotegerin (OPG) expression and a decrease in receptor activator of nuclear factor kappa-B ligand (RANKL) expression33,34. The specificity of romosozumab lies in its binding to sclerostin, a modulator of Wnt/β-catenin signaling. This interaction with sclerostin leads to the interaction of LRP5/6 and LRP4, effectively blocking Wnt binding to its receptor. This process involves coiled transmembrane receptor binding, GSK-3 phosphorylation by Zβ-catenin, and subsequent β-catenin degradation through ubiquitin-mediated proteolysis. This cascade ultimately promotes the expression of osteogenic genes35. In a comparison of the osteogenic effects of the GOM and GORoM hydrogels, several parameters, including ALP staining, ARS staining, mRNA expression (collagen type I [Col-1], osteocalcin [OCN], Runt-related transcription factor 2 [RunX2], and osteopontin [OPN]), and protein expression (RunX2 and OPN), were notably elevated in the GORoM group. This trend was also observed for the quantitative analysis of ALP and ARS staining as well as for the assessment of osteogenic proteins (Fig. 4AâJ). Collectively, these findings confirm that the beneficial in vitro osteogenic effects of both the GOM and GORoM hydrogels can be attributed to the sustained release of romosozumab through the utilization of MBGNs.
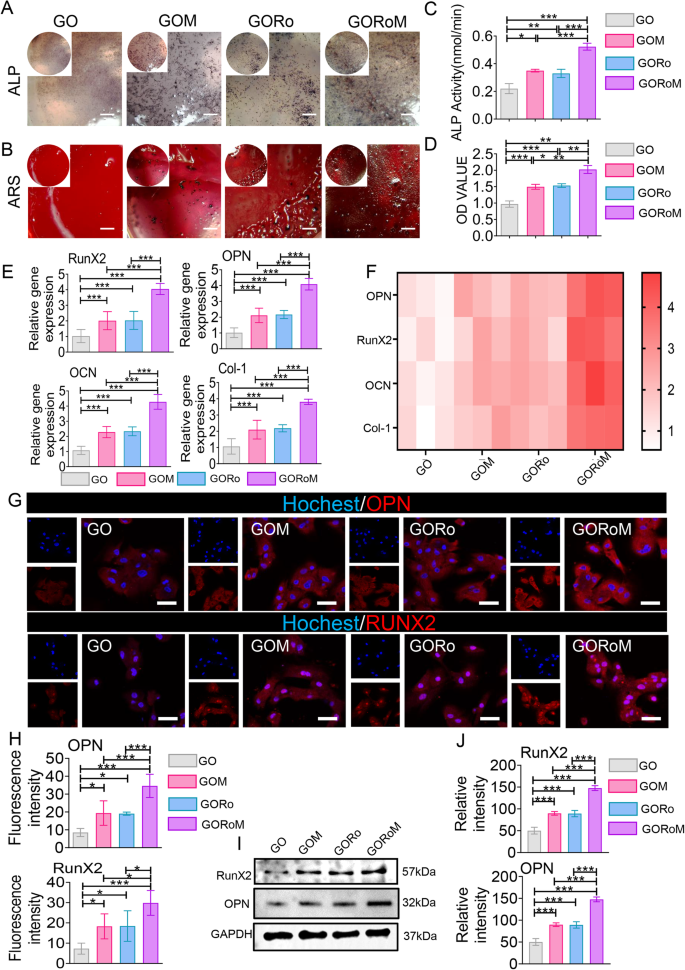
A ALP staining of BMSCs cultured on each adhesive for 7 days (scale bar: 100 μm). B ARS staining of BMSCs cultured on each adhesive for 14 days (scale bar: 100 μm). C Quantitative analysis of ALP staining (nâ=â3). D Quantitative analysis of ARS staining (nâ=â3). E, F The expression of osteogenesis-related genes, including OPN, RunX2, OCN, and Col-1, was estimated by RTâqPCR after BMSCs were cultured on the hydrogel surface for 14 days (nâ=â3). G Immunofluorescence images showing OPN and RunX2 staining of BMSCs cultured on the hydrogel surface for 14 days (scale bar: 50 μm). OPN and RunX2 were stained red, and the nuclei were stained blue. H Quantitative analysis of immunofluorescence staining (nâ=â3). I The protein expression of RunX2 and OPN was evaluated by Western blotting. J Quantitative analysis of the Western blotting data (nâ=â3). *pâ<â0.05, **pâ<â0.01, and ***pâ<â0.001.
The inhibitory effect of sample extracts on cell osteoclastic differentiation
We then explored how the hydrogels influenced osteoclast differentiation by employing BMDMs cultured in conditioned medium supplemented with osteoclast differentiation factors such as M-CSF and RANKL. RANKL, produced by osteocytes and osteoblasts, initiates the activation of the receptor activator of nuclear factor-B (RANK) on preosteoclasts, facilitating their transformation into mature osteoclasts33. TRAP staining was conducted on BMDMs following incubation with each GO-based adhesive to confirm the ability of each hydrogel to inhibit osteoclast formation. As shown in Fig. 5A, B, the GORo group displayed fewer TRAP-positive multinucleated cells after 7 days of culture than did the GO hydrogel group. Additionally, romosozumab resulted in a diminished actin ring size, indicating that romosuzumab significantly inhibited osteoclast maturation (Fig. 5CâE). As a result of the romosozumab-sclerostin interaction, Wnt/β-catenin signaling stimulates osteoblast and osteocyte survival, thereby inhibiting osteoclast maturation via enhanced OPG expression and reduced RANKL expression within osteoblasts and osteocytes33. Notably, GOM exhibited continuous romosuzumab release to prolong this effect, as the smallest TRAP-positive multinucleated cells and actin rings were observed in the GORoM group (Fig. 5AâE). Taken together, the above findings underscore the ability of our GORoM hydrogel to effectively suppress osteoclastic differentiation.
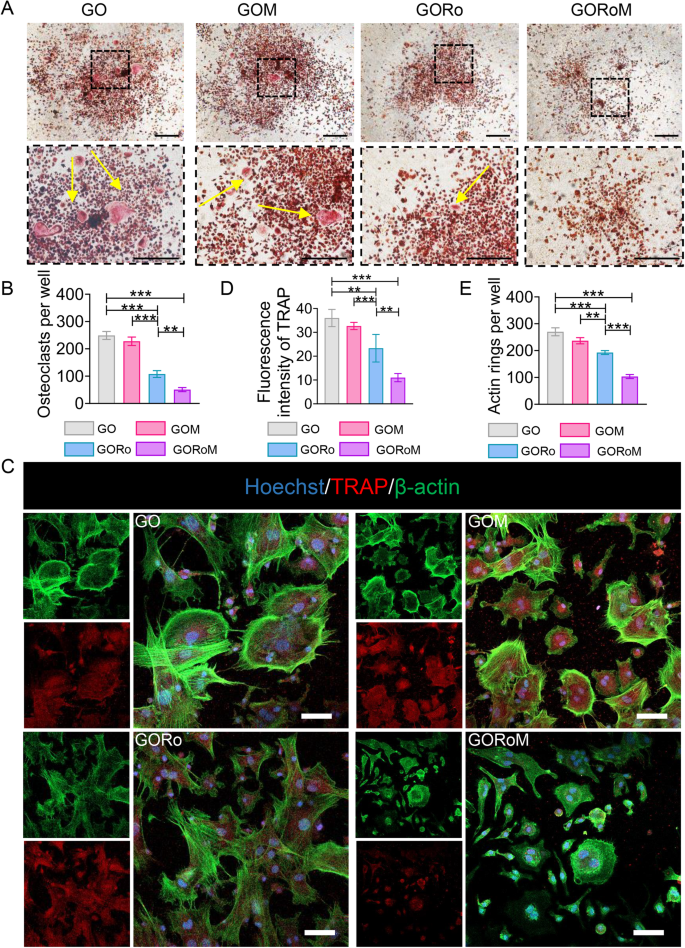
A TRAP staining of BMDMs cultured on different adhesive surfaces in RANKL-containing medium for 7 days (scale bar: 100 μm). B Quantitative analysis of TRAP-positive cells (nâ=â3). C Immunofluorescence staining for actin rings (green) and TRAP (red) on Day 7 (scale bar: 100 μm). D Quantitative analysis of the fluorescence intensity of TRAP (nâ=â3). E Quantitative analysis of the actin ring (nâ=â3). *pâ<â0.05, **pâ<â0.01, and ***pâ<â0.001.
Assessment of bone formation and bone resorption in vivo
Prior in vivo findings underscore the dual capacity of the GORoM hydrogel to promote osteogenesis while inhibiting osteoclastogenesis, which is essential for restoring balanced bone formation (impacted by osteoblasts) and bone resorption (influenced by osteoclasts). Consequently, we sought to restore dysregulated bone homeostasis in vivo using an OVX rat model, a prevalent model in osteoporotic bone fracture studies36,37,38. After 12 weeks of ovariectomy-induced osteoporosis, a femur fracture rat model was established, and treatment involving the GO-based adhesive or no treatment was administered. At 6 and 12 weeks post-implantation, the rat femoral tissue was subjected to microcomputed tomography (micro-CT) analysis (Fig. 6A). The 3D-reconstructed images at 6 weeks exhibited no apparent fracture displacement across the three GO-based adhesive groups, indicating the ability of each adhesive to support early-stage fracture fixation. After 12 weeks, a fracture line could still be observed in the GO and GOM groups, while nearly complete fracture healing was evident in the GORoM group (Fig. 6B). Indicators such as bone mineral density (BMD), bone volume/tissue volume (BV/TV), and trabecular thickness (Tb.Th) improved throughout the 6-week period, indicating effective healing potential across all groups. At 12 weeks, the osteogenic indices (BMD, BV/TV, and Tb.Th) peaked in the GORoM group (Fig. 6C), highlighting the robust osteogenic effect of the GORoM hydrogel.
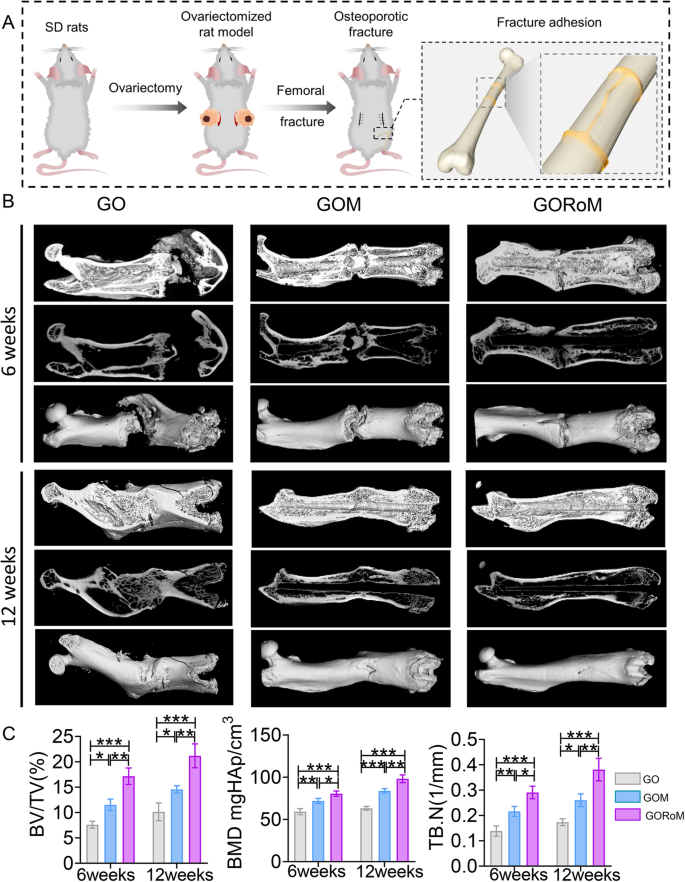
A Schematic showing the OVX rat femoral fracture model and the therapeutic effect of the GORoM hydrogel on the OVX rat femoral fracture model. B Micro-CT 3D reconstruction of a rat femur at 6 and 12 weeks. C BV/TV, BMD, and Tb.N, which reflect the bone volume and mineral density in the defect region, were quantitatively analyzed (nâ=â3). *pâ<â0.05, **pâ<â0.01, and ***pâ<â0.001.
Then, decalcified histological sections underwent chemical (HE, Masson) and immunohistochemical staining, which included bone formation (Col-1, OCN) and resorption (TRAP)-related proteins to assess the regenerative process at the cellular and molecular levels. Consistent with the radiological findings, HE staining at 6 weeks revealed nonunion in all four groups (Fig. 7A). The GORoM group exhibited more newly synthesized collagen tissue (blue staining) at the fracture site, along with a heightened aggregation of osteoblasts. Consequently, new bone (red staining) formed along the edge of the bone defect (Fig. 7C). Masson staining revealed that the newly formed bone had evolved into a honeycomb-like trabecular bone structure at 12â+â6 weeks (Fig. 7B, D). Active osteoblasts clustered along the edge of the new bone, and the GORoM group exhibited no visible fracture line, accompanied by notable callus enlargement, indicating successful repair. During the evaluation of osteoblast and osteoclast activity, Col-1, a vital structural constituent of new bone tissue expressed during osteoblast differentiation, exhibited increased expression in newly grown trabecular bones in the GORoM group at both 6 and 12 weeks (Fig. 8A). Similarly, OCN (pivotal for staining osteoblasts and osteoids and expressed during bone mineralization39) exhibited elevated expression in the GORoM group compared to the other two groups at 6 and 12 weeks (Fig. 8B). These results were further verified by quantitative analysis (Fig. 8C). Importantly, TRAP staining and quantitative analysis (Fig. 9A, B) revealed numerous RANKL-positive osteoclasts (claret-stained) at the fracture site in the GO group, while no osteoclasts were observed in the GORoM group. These findings confirm the ability of GORoM to suppress osteoclast activity and effectively reverse bone loss in OVX rats.
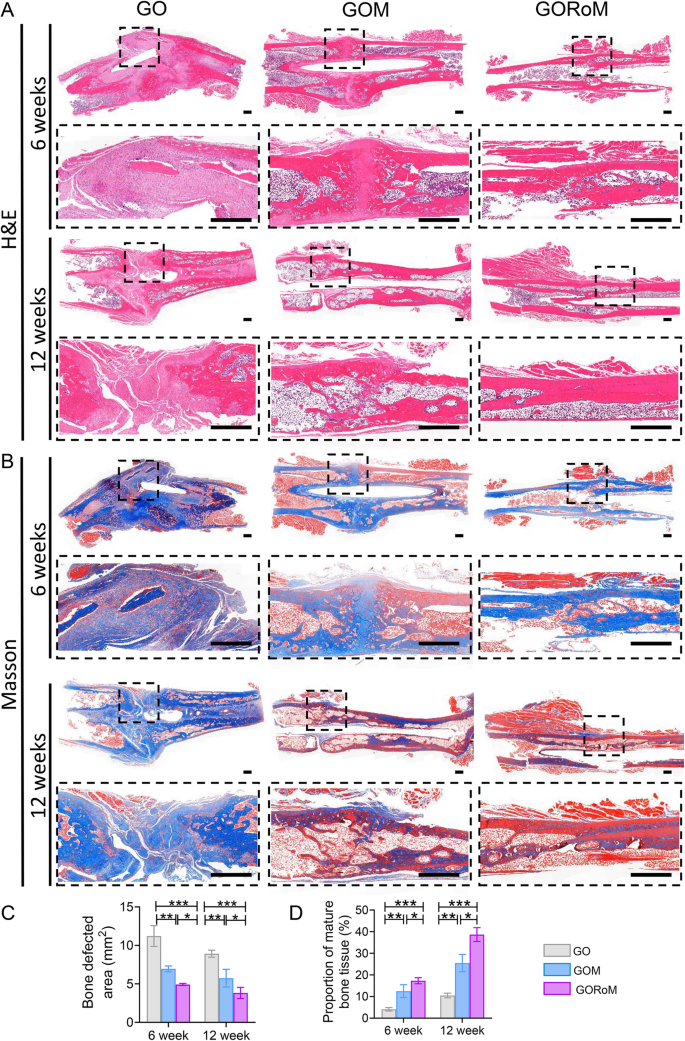
A HE staining and B Masson staining of defect areas after 6 and 12 weeks of treatment at low and high magnifications, respectively (scale bar: 500 μm). C Quantitative analysis of the bone defect area after 6 and 12 weeks (nâ=â3). D The quantitative proportion of mature bone tissue (stained with red) after 6 and 12 weeks (nâ=â3). *pâ<â0.05, **pâ<â0.01, and ***pâ<â0.001.
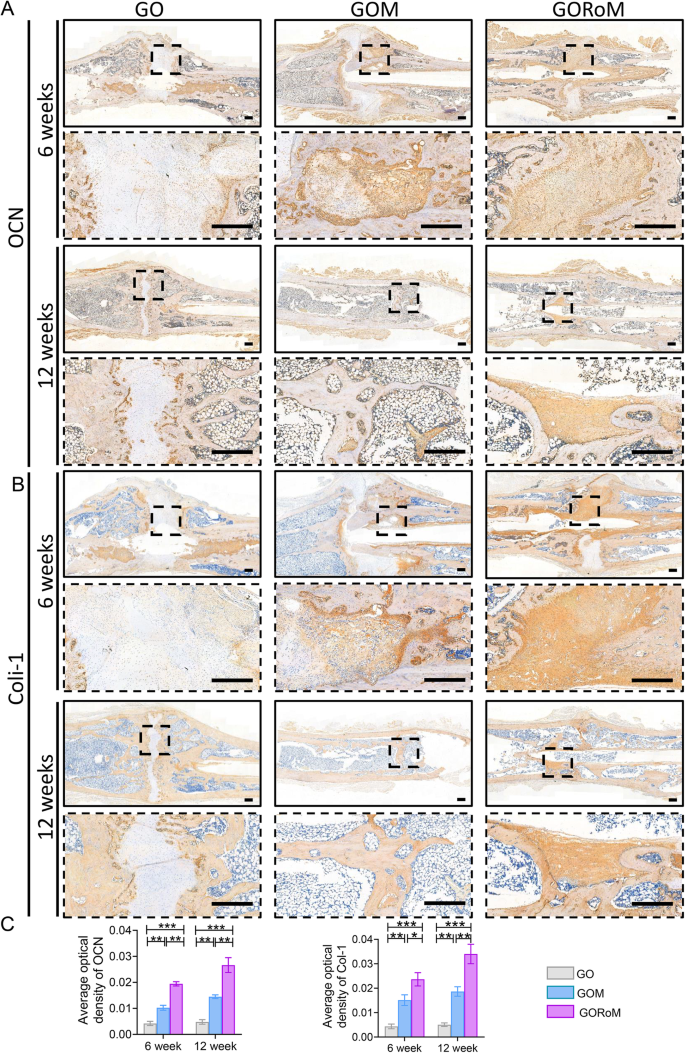
A Immunohistochemical staining of OCN in bone defects at 6 and 12 weeks (scale bar: 500 μm). B Immunohistochemical staining of Col-1 in bone defects at 6 and 12 weeks (scale bar: 500 μm). C Quantitative analysis of OCN and Col-1 expression at 6 and 12 weeks (nâ=â3). *pâ<â0.05, **pâ<â0.01, and ***pâ<â0.001.
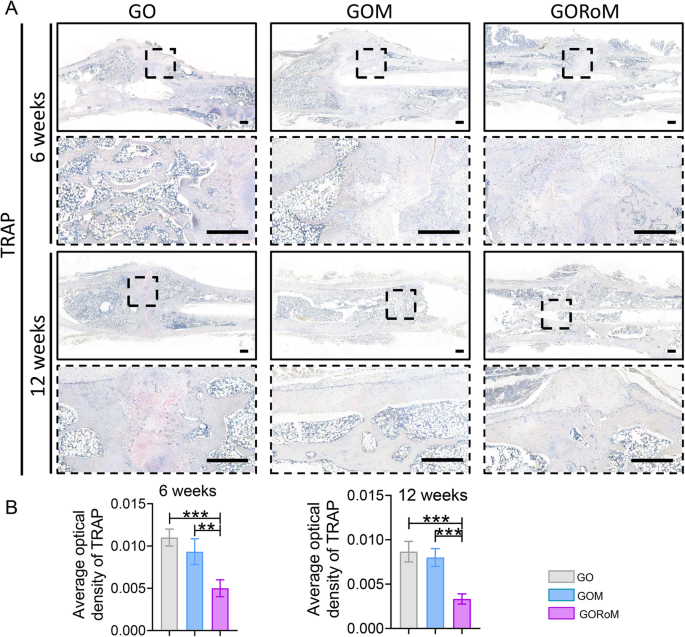
A Immunohistochemical staining of TRAP (scale bar: 500 μm). B Quantitative analysis of TRAP staining at 6 and 12 weeks (nâ=â3). *pâ<â0.05, **pâ<â0.01, and ***pâ<â0.001.
In summary, the GORoM hydrogel adopts a multifaceted approach to promote osteoporotic fracture regeneration by combining fracture fixation with rebalancing the bone remodeling process to strengthen osteogenesis while dampening bone resorption activity to achieve comprehensive osteoporotic fracture regeneration.