Selective transport of metal ions into synthetic cells
The specific transport of non-membrane-permeable metal ions into lipid-based vesicles remains a challenge. Although membrane pores (for example, α-haemolysin) let in all molecules below a certain size, they also lead to the loss of encapsulated small molecules. In this study, we proposed the use of ionophores with high specificity for individual metal ions to enable differential transport into GUVs. The ionophores facilitated diffusion along concentration gradients while maintaining separation of intracellular and extracellular content32,33. Specifically, we investigated the transport of Ni2+ with ionophore A (meso-tetraphenylporphine-4,4′,4″,4″′-tetracarboxylic acid)34, of Cu2+ with ionophore B (o-xylylen-bis-(N,N-diisobutyldithiocarbamat))35 and of Ca2+ with ionophore C (ionomycin)36 (Fig. 1b).
To determine the specificity of these ionophores in metal ion transport across the membrane, we loaded GUVs (1-palmitoyl-2-oleoyl-glycero-3-phosphocholine (POPC)) with 0.1 mol% DiD (1,1′-dioctadecyl-3,3,3′,3′- tetramethylindodicarbocyanine, 4-chlorobenzenesulfonate salt) membrane dye (shown in red) and the metal ion-sensitive fluorescent dye Rhod2 or its Ca2+ complex (shown in green) (both Rhod2 and the complex are membrane impermeable). We monitored the transport of externally added Ni2+, Cu2+ and Ca2+ ions with ionophores A, B or C into the GUVs by using confocal fluorescence microscopy (Fig. 1c). Rhod2 itself is non-fluorescent but becomes fluorescent upon complexing with Ca2+ ([Ca2+-Rhod2]), which is quenched in the presence of heavy metal cations such as Ni2+ or Cu2+ ions37. The fluorescence of [Ca2+-Rhod2] in the GUVs decreased upon addition of ionophore A, but not B or C, in the presence of outer Ni2+ ions (Fig. 1c and Supplementary Fig. 1a). Similarly, the addition of ionophore B quenched the [Ca2+-Rhod2] fluorescence in the presence of external Cu2+ ions, whereas ionophores A and C did not show any effect (Fig. 1c and Supplementary Fig. 1b). The internal fluorescence of Rhod2-loaded GUVs increased in the presence of external Ca2+ ions upon addition of ionophore C, whereas no change was observed with ionophores A or B (Fig. 1c and Supplementary Fig. 1c). Ion transport into the GUVs depended on ionophore concentration as measured in bulk populations of GUVs with a plate reader (Fig. 1d and Supplementary Fig. 2a–c). The optimal concentrations for ionophores A, B and C were 20 µM, 5 µM and 1 µM, respectively, and these concentrations were used throughout the study unless otherwise specified. The transport of each ion with its corresponding ionophore was rapid, with changes in Rhod2 fluorescence observed within 5–10 min of ionophore addition in the plate reader measurements (Fig. 1e) and at the level of single GUVs under the microscope (Supplementary Fig. 3a–c). Overall, these findings demonstrate the high selectivity of ionophore A for Ni2+, ionophore B for Cu2+ and ionophore C for Ca2+ for transport into GUVs, other metal ions being excluded.
Activation of metalloenzymes in synthetic cells
Next, we aimed to translate the selective transport of each metal ion into the activation of distinct enzymatic activities in the GUV. To achieve this, we proposed encapsulating dormant apo-metalloenzymes that become catalytically active upon binding to their cognate metal ion cofactors. In the framework of this study, we chose three metalloenzymes as demonstrators: urease as a Ni2+ enzyme that decomposes urea into ammonia and carbon dioxide, resulting in an increased pH38; galactose oxidase (GaoA) as a Cu2+ enzyme that oxidizes d-galactose to d-galacto-hexodialdose and produces H2O2 (ref. 39); and phospholipase A2 (PLA2) as a Ca2+ enzyme that cleaves fatty acid chains of phospholipids, leading to the lysis of GUVs40. We chose these three enzymes on the basis of multiple requirements. First, our focus was on identifying three metalloenzymes with single metal cofactors that matched the specificity of the ionophores, ruling out metalloenzymes with other metal ion cofactors. Second, we looked for metalloenzymes that had distinct enzymatic activity that could be monitored in real time with available fluorescent sensors. Finally, we verified that the enzymes were stable in their apo-form and not denatured upon metal ion removal. To this end, for each of the three enzymes, we first produced the apo-forms and confirmed the activation of apo-GaoA and apo-urease in solution after addition of their metallocofactor (Supplementary Fig. 4a,b). In addition, we showed that low concentrations of ethylenediaminetetraacetic acid (EDTA, 30 µM), later included in the GUV preparation to avoid aberrant enzyme activation, do not alter enzyme activation upon addition of the metal ions (Supplementary Fig. 4c,d). Next, we evaluated the activation of each apo-metalloenzyme inside the GUV with selective metal ion transport. In GUVs loaded with apo-urease, the fluorescence of the co-encapsulated fluorescent pH indicator, 8-hydroxy-pyrene-1,3,6-trisulfonic acid trisodium salt (HTPS; urease sensor, shown in cyan), was initially low in the presence of the membrane-permeable substrate urea and external Ni2+ ions, as observed over 20 min (Fig. 2a). After we added ionophore A, the fluorescence of the urease sensor increased as Ni2+ ions entered the GUVs and activated the urease, producing ammonia and increasing the internal pH. The pH in the GUVs rose from 7.4 to above 9 within 20 min, as observed by tracking the same GUV over time (Fig. 2b and Supplementary Fig. 5) and by analysing a population of GUVs after 20 min (Supplementary Fig. 6a). Notably, we observed that few GUVs deformed and even formed interconnected vesicles as a result of elevated internal pH and osmotic pressure, as also reported in some previous studies6,38,41 (Supplementary Fig. 7 and Supplementary Movie 1).
a, Schematic and CLSM images of the same apo-urease-loaded GUV (membrane shown in red) in the presence of Ni2+, increasing its intracellular pH (urease sensor shown in cyan) upon addition of ionophore A owing to Ni2+ transport into the GUV and activation of urease. b, The fluorescence increase inside GUVs over time in a. The error bars with fill area indicate the s.d. of the average intensities for nGUV = 10. c, Schematic and CLSM images of the same apo-GaoA-loaded GUV in the presence of Cu2+, producing H2O2 (GaoA sensor shown in green) upon addition of ionophore B due to Cu2+ transport into the GUV and activation of GaoA. d, The fluorescence increase inside the GUV over time in c. The error bars with fill area indicate the s.d. of the average intensities for nGUV = 10. e, Schematic and CLSM images of apo-PLA2-loaded GUV, lysing upon addition of ionophore C owing to Ca2+ transport and activation of PLA2. f, The GUV area over time. The error bars with fill area indicate the s.d. of the average intensities for nGUV = 3. Scale bars, 10 µm. Individual GUVs were tracked over time in all experiments.
Source data
To demonstrate the activation of apo-GaoA inside GUVs via Cu2+ transport, we loaded apo-GaoA, galactose (membrane impermeable) and horseradish peroxidase (HRP) into GUVs while Cu2+ and Amplex red were present in the external solution (Fig. 2c). After 20 min of incubation, the GUVs exhibited no notable increase in their internal fluorescence from the GaoA sensor (shown in green; H2O2 oxidizes non-fluorescent Amplex red (membrane permeable) to fluorescent resorufin (membrane impermeable) catalysed by HRP). Yet, upon initiation of the Cu2+ transport with ionophore B, the fluorescence signal from the GaoA sensor in the GUV increased over 20 min (Fig. 2d). Complementarily, population-level analysis (Supplementary Fig. 6b) and the detection of H2O2 with a chemiluminescence assay (Supplementary Fig. 8) provided further evidence of increased GaoA activity following the addition of ionophore B.
To demonstrate the activation of apo-PLA2, we encapsulated the apo-enzyme into GUVs, which remained stable in the presence of external Ca2+ after 20 min (Fig. 2e). On addition of ionophore C, the GUV membranes started to break after approximately 10 min (Fig. 2f and Supplementary Movie 2), eventually leading to the lysis of the GUV into small micelles. In the analysis of multiple GUVs, a visible reduction in the area of GUVs was observed (Supplementary Fig. 6c). In all these experiments, we first added the metal ion and second the ionophore after 20 min equilibration to activate the apo-enzymes encapsulated in the GUVs, but this worked equally well when the ionophore was added first and the metal ion second after 20 min (Supplementary Fig. 9). Moreover, for all three enzymes, control experiments showed that there was no unspecific enzyme activation without added ionophores (Supplementary Fig. 11). These results exemplify the activation of different apo-metalloenzymes with distinct metal ion cofactors within GUVs through selective ionophore-mediated transport, resulting in diverse outcomes, such as pH increase, H2O2 generation and vesicle lysis.
Differential enzyme activation in a synthetic cell
We next investigated whether the selective activation of a single metalloenzyme is possible in a pluripotent synthetic cell with multiple dormant enzymes. For this purpose, we loaded GUVs with all three apo-enzymes and assessed whether it is possible to activate one specific enzyme with all three metal ions present in the environment by using the corresponding ionophore (Fig. 3a). These GUVs (shown in red) also contained the necessary components for the urease sensor (shown in cyan) and GaoA sensor (shown in green), as well as all the encapsulated and externally added substrates. On addition of the Ni2+-selective ionophore A, we observed an increase in the fluorescence signal from the urease sensor, leading to fate A, characterized by an elevated internal pH in the pluripotent synthetic cell (Fig. 3b,c). At the same time, there was no increase in fluorescence from the GaoA sensor, and the GUVs remained stable, indicating the selective activation of urease and not the other enzymes. In contrast, when the Cu2+-selective ionophore B was added to the pluripotent GUVs, only the fluorescence signal from the GaoA sensor and not the urease sensor increased, leading to fate B, characterized by the production of H2O2 (Fig. 3d,e). It is worth noting that the gradual decrease in the GaoA sensor signal inside the GUV and increased background at later stages might be due to the photobleaching of resorufin inside the GUV and the residual activity of enzymes outside the vesicles as well as the non-catalysed photooxidation of Amplex red to resorufin. Finally, upon addition of the Ca2+-selective ionophore C to the pluripotent GUVs, the GUVs ruptured over time, leading to fate C, characterized by GUV lysis as a result of the specific activation of PLA2 (Fig. 3f and Supplementary Movie 3). Moreover, when we tracked the fluorescence of the urease and GaoA sensors after adding ionophore C, we observed no increase in fluorescence in either channel (Supplementary Fig. 10), presumably because the loaded galactose and the urease sensor leaked from the GUVs. Notably, incubating the pluripotent GUVs solely with the three metal ions failed to induce enzyme activation (Supplementary Fig. 11). Furthermore, we confirmed that the transport of metal ions into GUVs by the specific ionophore remained unaffected in the presence of the other spectator ions (Supplementary Fig. 12). Overall, we successfully demonstrated the ability to control the activation of a specific metalloenzyme within pluripotent GUVs, depending on the metal ion transporter.

a, Schematic of a pluripotent synthetic cell loaded with three apo-metalloenzymes (apo-urease, apo-GaoA and apo-PLA2) differentiated with different ionophores in the presence of three external metal ions (Ni2+, Cu2+ and Ca2+). b,d,f, CLSM images of pluripotent GUV (membrane shown in red) loaded with urease sensor (shown in cyan) and GaoA sensor (shown in green) after adding ionophore A, resulting in a pH increase (b); ionophore B, resulting in H2O2 production (d); or ionophore C, resulting in GUV lysis (f). Scale bars, 10 µm. c,e, Fluorescence change for the urease sensor and GaoA sensor inside GUV in b (c) and in d (e). The error bars with fill area indicate the s.d. of the average intensities for nGUV = 10. Individual GUVs were tracked over time in all experiments.
Source data
Ion transport capacity depends on ionophore sequence
In pluripotent cells, the operation of different pathways is not independent; instead, there is extensive cross-regulation in which the activation of one pathway can suppress or activate others. Consequently, the temporal sequence of differentiation signals is crucial for determining the final cell fate. In this respect, we investigated whether it is possible to sequentially transport different metal ions independent from each other into a pluripotent GUV, or if there are feedback mechanisms that alter the transport of later metal ions.
To achieve this, we initially examined the effects of sequentially adding ionophores on the transport of their specific metal ions. As previously demonstrated, ionophore A transports external Ni2+ ions into GUVs based on the quenching of [Ca2+-Rhod2] fluorescence (shown in green), which was defined as 100% (Fig. 4a). Yet, when GUVs were first exposed to ionophores B or C for 20 min and then to ionophore A, the relative ion transport for Ni2+ ions decreased to 31% and 45%, respectively (Fig. 4b and Supplementary Fig. 13a). The impact of spectator ionophores was even more pronounced when ionophore A was added as the third step after two prior 20 min incubation steps with ionophores B and C. In this case, there was almost no Ni2+ transport (below 20%), and the diminished Ni2+ transport with ionophore A was independent of the type and order in which the spectator ionophores were added beforehand (B and then C, or C and then B). Likewise, the presence of other ionophores influenced the transport of external Cu2+ ions with ionophore B. The prior addition of one (A or C) or two ionophores (A and then C, or C and then A) decreased the relative transport below 25% in all cases, compared with when ionophore B was added alone (Fig. 4c,d and Supplementary Fig. 13b). Similarly, the addition of ionophore A and B negatively impacted the transport of Ca2+ into GUVs with ionophore C, visualized as an increase in [Ca2+-Rhod2] fluorescence (Fig. 4e). The earlier addition of one (A or B) or two ionophores (A and then B, or B and then A) decreased the relative transport below 50% in all cases, compared with when ionophore C was added first (Fig. 4f and Supplementary Fig. 13c). Thus, the order in which the ionophores were added determined which metal ions entered the GUVs and to what extent, and the prior addition of one ionophore inhibited transport with subsequent ionophores in all three cases.

a,c,e, Representative CLSM images of a GUV (membrane shown in red) loaded with [Ca2+-Rhod2] or Rhod2 (shown in green) before and after sequential addition of up to three ionophores (20 µM A, 5 µM B and 1 µM C) in 20 min intervals in the presence of external NiCl2 (a), CuCl2 (c) or CaCl2 (e). Scale bars,10 µm. b,d,f, The relative ion transport of Ni2+ in a (b), Cu2+ in c (d) and Ca2+ in e (f). The error bars indicate the s.d. of the average intensities for nGUV = 10. Statistical significance is determined by two-tailed non-paired Student’s t-tests (***P < 0.001, **P < 0.01, *P < 0.05; n.s., non-significant). g, The relative ion transport of each metal ion in the presence of different competitor ionophores added 20 min before the cognate ionophore (20 µM A, 5 µM B and 1 µM C). The error bars indicate the s.d. of the average intensities for nGUV = 10. Randomly picked GUVs were analysed in each sample.
Source data
These observations raise the question of what the mechanism is for the negative feedback of the ionophores among each other. In further experiments, we observed that, when the GUVs were first incubated with one of the metal ions (Ni2+, Cu2+ or Ca2+) and then a cocktail of the three ionophores was added at once, none of the metal ions was transferred into the GUVs (Supplementary Fig. 14a–c). This observation indicates that the insertion of spectator ionophores decreases the ability of the cognate ionophore to transport its metal ion across the membrane and that there is no kinetic preference of one ionophore over the other inserting faster into the GUVs. Moreover, we investigated the effect of different concentrations of the first ionophore on transport with subsequent ionophores (Fig. 4g and Supplementary Figs. 15 and 16). In each case, we observed that, when the first ionophore was added at working concentration (20 µM, 5 µM and 1 µM for ionophores A, B and C, respectively), the transport with the second ionophore decreased drastically. These results suggest that the functionality of the second ionophore in the GUV membrane depends closely on the amount of the other ionophores present in the membrane.
MD simulations provide microscopic dynamic insight
To gain a better molecular picture of the interactions between the ionophores inside the GUV membrane and understand how they negatively influence each other’s activity, we conducted molecular dynamics (MD) simulations for all three ionophores in a POPC bilayer. In these simulations, we considered the comparatively rigid and planar ionophore A (C48H30N4O8, C4 symmetric), the more flexible ionophore B (C26H44N2S4, C2 symmetric) and the larger, flexible and symmetry-free ionophore C (C41H72O9). As can be seen in the time evolution of the z coordinate of the centre of mass (COM) of all three ionophores within the membrane, ionophores A and B switched several times between the two leaflets within the 2,000 ns simulation time, albeit with many more transitions for ionophore B than ionophore A (Fig. 5a,b). Interestingly, the orientation of ionophore A was parallel to the membrane surface when close to the head group and otherwise orthogonal inside the lipid bilayer, presumably allowing the transport through the membrane, but no such preferred orientation was seen for ionophore B (Supplementary Fig. 17). Furthermore, no transitions were observed for ionophore C within the same simulation time of 2,000 ns (Fig. 5c). Transitions of ionophore C may happen at longer time scales, but these become too demanding in terms of computational time. However, one may investigate the impact of ionophore C on the other species within the lipid bilayer in these simulations.

a–c, The time-dependent position along the membrane normal (z coordinate) of the COM of a single ionophore for the three ionophores A (a), B (b) and C (c), respectively. The position of the centre of the membrane is at z = 0, and the approximate average positions of the lipid head group in the two leaflets are represented by dashed red lines. On the right side are some snapshots. d, Similar to a but with NA = 2. e, Similar to a but with NA = NB = 1. f, Similar to a but with NA = NC = 1. In d–f, the traces of ionophores A, B and C are shown in (light and dark) blue, green and orange, respectively, and each ionophore is shown individually. g, The probability of finding ionophore A at a specific z value in the intensity-sensitive representation (Methods). For the case of two ionophores, only those snapshots where both ionophores are in the same leaflet contribute. h, The RDFs for the compositions, shown in d–f. A snapshot is shown for the specific configuration of ionophore A corresponding to the peak at ~2.2 nm for A–A pairs. i, Similar to a but with NA = NB = 2.
Additional information is gained in considering the mutual impact of ionophores on each other. For this purpose, trajectories of systems with two ionophores were considered, namely, the combination of ionophore A with any of the ionophores A, B or C, involving interactions between like and unlike ionophores (Fig. 5d,f). This corresponds to two-dimensional densities of 0.05 ionophores nm−2 (2 ionophores in 106 POPC lipids). To improve the statistical analysis, we generated each of the trajectories with unlike ionophores twice in independent simulations. The number of transitions between the two leaflets of the membrane for ionophore A was 4 for single ionophore A (NA = 1), 3 in the presence of a second ionophore A (NA = 2), 0.5 in the presence of one ionophore B (NA = NB = 1) and 0.5 in the presence of one ionophore C (NA = NC = 1), using the average over both trajectories for the final two cases. Thus, there were hardly any transitions of ionophore A when a second unlike ionophore was present, and this effect was weaker for the addition of a like ionophore. On closer inspection, it became evident that ionophore A was shifted to the outer part of the membrane when a second unlike ionophore was present as compared with the case of a single ionophore A (Fig. 5e,f). This shift is probably a direct consequence of the interaction among the ionophores and naturally explains the emergence of a reduced transition rate of ionophore A. A more quantitative account of this observation is shown in Fig. 5g, where the position of ionophore A is clearly shifted to the periphery when ionophore B or C is present in the same leaflet of the membrane. In contrast, the presence of a second ionophore A had no noticeable impact on ionophore A’s position in the membrane, which is in agreement with the remaining ability to transition between the different leaflets of the membrane.
For all three pairwise interactions (A–A, A–B and A–C), the radial distribution function (RDF) displayed considerable deviations from unity around 1 nm, consistent with interactions among the ionophores within the same leaflet (Fig. 5h). However, the differences are not as pronounced and much longer simulations would be required, involving an averaging over different interaction patterns, to obtain fully convergent behaviour of the RDF. There were only weak interactions beyond 1 nm except for the peak at ~2.2 nm for A–A pairs, which corresponds to a specific edge-to-edge binding where each ionophore A is in a different leaflet (Fig. 5h).
The corresponding pairwise interactions involving ionophores B and C were also considered (Supplementary Fig. 18). Despite the important deviations in the RDF from unity for the B–B and B–C pairs (Supplementary Fig. 19), the number of transitions of ionophore B was not notably perturbed by the presence of a second ionophore (16 transitions for NB = 1, 12 transitions for NB = 2, 17 transitions for NB = NA = 1 and 13 transitions for NB = NC = 1). However, there was a substantial inward shift of ionophore B in the presence of ionophore C (Supplementary Fig. 20a), which may reduce the binding probability to a metal ion at the surface.
Further information about the influence of ionophore density in the membrane on ionophore mobility can be gained from studying systems with more than two ionophores. Indeed, at higher densities of ionophores A (NA = 4), transitions between the leaflets were no longer possible (Supplementary Fig. 21a) due to considerable clustering among the ionophores (Supplementary Fig. 22). Similarly, clustering effects were observed for ionophore B (Supplementary Fig. 23), although, in contrast to ionophore A, they were only in the form of transient loosely packed structures. Nevertheless, for NB = 4, the number of transitions reduced to 7 compared with the 16 transitions for NB = 1 (Supplementary Fig. 21b). Lastly, in systems that included a mixture of multiple ionophores, that is, NA = NB = 2, the switching between the two leaflets for ionophore A was also strongly reduced (one transition compared with three for NA = 2 and four for NA = 1) (Fig. 5i), due to expected interactions between ionophores A and B.
Overall, the MD simulations of ionophore A with either ionophore A, B or C indicate noticeable interactions among the ionophores, which impact the transition rate between the two leaflets of the membrane and are likely to give rise to less effective metal ion transport. Effects are also seen for ionophore B with either ionophore B or C. Moreover, the identified interactions between the different ionophores and their tendency to form clusters within the membrane are a possible molecular origin of the negative effect that the ionophores have on each other’s ability to transport metal ions across the membrane.
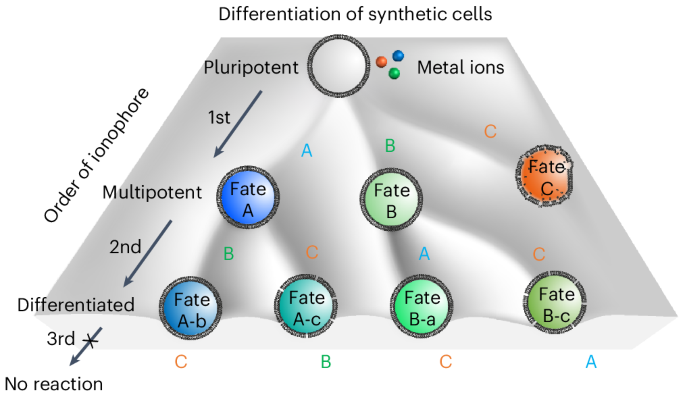
Ionophore sequence determines fate of synthetic cells
In pluripotent cells, the commitment towards one fate means gaining new capabilities while simultaneously losing other dormant functions. The pluripotent cell makes these decisions on the basis of the spatiotemporal context of different cues in its environment and differentially up- or downregulates functions accordingly. Moreover, the process of differentiation can go through multiple steps, in which the cell first converts to a more specified multipotent cell and in a second step reaches a terminally differentiated state. Analogously, we envisioned that memory of the GUVs for the sequence in which the ionophores were added provides a basis to build pluripotent synthetic cells, which can be differentiated in multiple steps. In other words, the first ionophore would lead to commitment to a certain fate (fate A, increased internal pH; fate B, H2O2 production; or fate C, lysis) and convert the pluripotent into a multipotent GUV. Subsequently, the second ionophore can terminally differentiate the GUV by relying on the residual activity of the second ionophore.
To test this multistep differentiation towards different cell fates, we placed the above-described pluripotent GUVs (membrane shown in red) with the co-encapsulated apo-enzymes (apo-urease, apo-GaoA and apo-PLA2), substrates and urease and GaoA sensors (shown in cyan and green, respectively) or the membrane-impermeable dye, sulfo-Cy5 (shown in orange), into environments with external Ni2+, Cu2+ and Ca2+ ions.
Upon addition of ionophore A, the signal from the urease activity sensor increased over the course of 20 min owing to the increase in internal pH, that is, fate A (Fig. 6a–d). Depending on which ionophore was added second, the subsequent response of the GUVs differed. If ionophore B was added second, the signal from the GaoA sensor increased slightly, showing low levels of H2O2 generation, and this was classified as fate A-b (Fig. 6a,b). When ionophore C was added to these GUVs as the third ionophore, there was no further change, representing a terminally differentiated GUV that is unresponsive to further inputs. In contrast, if ionophore C was added second, there was no response from the GaoA sensor and the GUVs did not lyse, but they became leaky due to partial PLA2 activation as observed through the release of loaded sulfo-Cy5 (shown in orange), resulting in fate A-c (Fig. 6c,d). Subsequently, the addition of ionophore B as the third one did not activate GaoA activity.

a,c,e,g, Representative CLSM images of GUVs (membrane shown in red) loaded with three apo-metalloenzymes in the presence of all three metal ions as different ionophores were added every 20 min. Order of ionophores is A-B-C (a), A-C-B (c), B-A-C (e) and B-C-A (g). The GUVs were loaded either with urease sensor (shown in cyan) and GaoA sensor (shown in green) or sulfo-Cy5 (shown in orange) for monitoring permeability. b,d,f,h, The mean fluorescence intensity of urease sensor (blue, nGUV = 10) and GaoA sensor (green, nGUV = 10), or sulfo-Cy5 (orange, nGUV = 10) inside GUVs in a (b), c (d), e (f) and g (h). i, Three-dimensional plots showing final states of GUVs exposed to the three ionophores in different orders (nGUV = 10). Individual GUVs either loaded with the urease and GaoA sensors or with sulfo-Cy5 were tracked throughout the experiment, and each GUV is from an independent sample. The error bars with fill area indicate the s.d. of the average intensities for nGUV = 10. Scale bars, 10 µm.
Source data
The outcome for the pluripotent GUV was different if ionophore B was added first instead of ionophore A. In this case, the signal from the GaoA sensor increased rapidly due to the transport of Cu2+ into the GUV and the activation of GaoA, resulting in fate B (Fig. 6e–h). If ionophore A was added second 20 min later, there was almost no increase in the urease sensor, fate B-a (Fig. 6e,f). Not observing an increase in pH may also be partially due to the further oxidation of d-galacto-hexodialdose to the corresponding acid with H2O2. Moreover, in this case, the addition of ionophore C as the third one had no consequence for GUV stability or membrane permeability. In contrast, if ionophore C was added second after ionophore B, the GUVs got leaky due to residual PLA2 activation, resulting in fate B-c (Fig. 6g,h).
The addition of ionophore C first leads to the lysis of the GUVs (fate C), as already demonstrated (Fig. 3f), but its addition as the second ionophore activates only low levels of PLA2. Consequently, the GUVs remain stable but become somewhat leaky (Fig. 6d,h and Supplementary Fig. 24). Finally, when ionophore C was added third, this resulted in insufficient Ca2+ influx into the GUVs and the GUVs remained intact and tight. When we increased the concentration of encapsulated apo-PLA2 to 500 nM and repeated the sequential addition of ionophores, we observed more prominent leakage of sulfo-Cy5 when ionophore C was added second (60% over 20 min) and minor leakage sulfo-Cy5 when ionophore C was added third (20% over 20 min). Most importantly, also in this case, only the addition of ionophore C first resulted in lysis (Supplementary Fig. 24). A three-dimensional plot of the final mean fluorescence intensities in the three channels delineates the four different outcomes of enzyme activation depending on the sequence in which the ionophores were added, with some overlap in fate B-a and fate B-c (Fig. 6i and Supplementary Figs. 25–27). Overall, the same pluripotent GUV was able to adapt five different fates, depending on the order in which the three ionophores were added, each step resulting in desensitization towards subsequent ones.