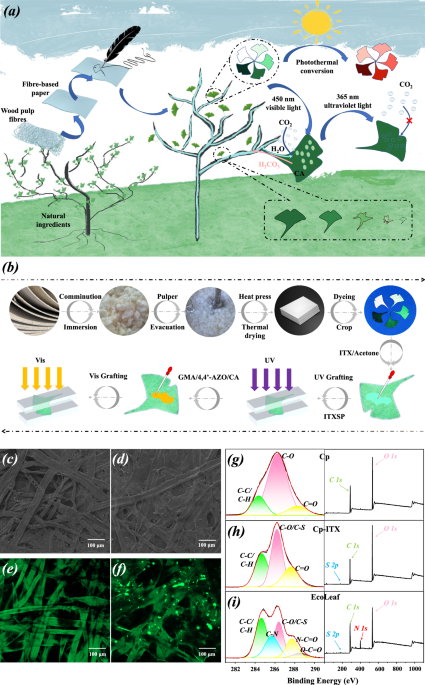
In this study, we constructed a biomimetic functionalized leaf, as depicted in Fig. 1a, b, beginning with a leaf drawn on paper. This artificial leaf, similar to natural leaves, integrates essential properties for the natural photosynthetic and carbon sequestration processes, including light capture, stomatal expansion and contraction, gaseous CO2 capture, product transportation, and biodegradability.
a Construction and performance of EcoLeaf; b The detailed preparation process of EcoLeaf; SEM maps of light-responsive 3D mesh matrix (Isopropyl thioxanthone, ITX) (c) before grafting vs. (d) after grafting of cellulose substrate; Fluorescence spectra of (e) EcoLeaf without encapsulated CA vs. (f) EcoLeaf encapsulated with FITC-CA under LCSM (The experiment was repeated independently three times with similar results); C 1s core-level spectra and full spectra of (g) Cellulose paper (Cp), (h) Cellulose paper-isopropyl thioxanthone (Cp-ITX), and (i) EcoLeaf.
A natural leaf primarily consists of the leaf blade plane, leaf flesh, and leaf veins30,31. The leaf plane is typically flattened to maximize sunlight reception32. Leaf pulp forms a three-dimensional reticular matrix responsible for organic matter production and storage through photosynthesis33. Leaf veins facilitate substance absorption, transportation, and distribution34. Inspired by this, in our study, we cut paper-drawn leaves into EcoLeaf planes. We covalently attached glycidyl methacrylate(GMA) with a C=C bond to both ends of the photoresponsive monomer 4,4â-Azodianiline(4,4â-AZO) through covalent conjugation. This GMA/4,4â-AZO was grafted onto the surface of the EcoLeaf planes, creating a photoresponsive three-dimensional reticulated matrix under visible light. The synthesis of the photoresponsive monomer GMA/4,4â-AZO was validated using NMR to confirm the epoxy and amino group reaction. The disappearance of the characteristic 4,4â-AZO amino proton peak (5.80 ppm) and the emergence of the nascent hydroxyl proton peak (5.2 ppm) of GMA/4,4â-AZO (Supplementary Fig. 1bâd) indicated the successful epoxy ring-opening addition reaction. This provided theoretical support for the attachment of the 3D mesh matrix to the cellulose substrate surface. A comparison between Fig. 1c, d demonstrates that graft polymerization of GMA/4,4â-AZO on the surface of kapok fibers fills the pores between fibers with a light-responsive reticulation matrix. This results in a denser fiber network, initial evidence of the successful construction of a three-dimensional reticulation matrix on the cellulose substrate.
Natural leaves rely on mitochondrial respiration for carbon sequestration, a process facilitated by enzyme complexes with high catalytic activity and selectivity. In our study, CA, known for its efficient CO2 turnover, served as the primary carbon sequestration organ in EcoLeaf. To confirm CAâs integration into the artificial leaf system, we encapsulated free CA with FITC staining and observed the distribution of FITC-CA within the artificial leaf system using LSCM. The green fluorescence in Fig. 1e is attributed to the chromophore group (-N=N-) in 4,4â-AZO, affirming the successful polymerization of the photoresponsive monomer on the cellulose surface. Additionally, the presence of numerous bright green fluorescent clusters in Fig. 1f, compared to Fig. 1e, can be ascribed to FITC-CA. To further visualize the 3D mesh matrix and CA, we explored the 3D distribution state of both in EcoLeaf using LSCM. Supplementary Fig. 2 displays the 3D tomograms of EcoLeaf and the 3D maps from different top-down view angles (0°, 60°, 90°). The encapsulation of the fiber surface by the 3D mesh matrix and the filling of the fiber pores can be clearly seen in the figure, reinforcing the successful construction of the 3D mesh cloth matrix. Additionally, the bright green fluorescence (FITC-CA) exhibits a relatively uniform distribution within the 3D mesh matrix. FITC contains a benzene ring and a thiocyanine group, which shift fluorescence emission peaks to longer wavelengths, thus intensifying fluorescence. By contrast, the relatively simple molecular structure of azobenzene in GMA/4,4â-AZO leads to weaker fluorescence. This preliminary evidence confirms the successful inclusion of CA in the EcoLeaf system. Furthermore, XPS analysis was conducted at various stages of EcoLeaf preparation to examine elemental and covalent bonding changes on the materialâs surface. In comparison to Cp (Fig. 1g), the C 1âs core spectrum of Cp-ITX and the full spectrum (Fig. 1h) show new C-S bonds and S introduced by the photoinitiator ITX. Additionally, the newly formed C-N bonds in Fig. 1i primarily originate from GMA/4,4â-AZO and CA. O-C=O and N-C=O are associated with the peptide bond and carboxyl group of CA, providing further evidence of successful EcoLeaf construction.
The mechanical properties of natural vane play a crucial role in its ability to withstand external forces. Good mechanical properties allow the blades to maintain their structural integrity and prevent them from breaking, getting damaged, or deforming under the influence of wind, gravity, rain, snow, and frost. Based on this, the mechanical properties of five different natural leaves and two EcoLeafs with different densities and thicknesses were investigated (Fig. 2a). It is evident that the mechanical properties of the paper used to make EcoLeaf are higher than those of most natural leaves in the left panel of Fig. 2b. Furthermore, based on the findings in Fig. 2b, c, it can be observed that the palm fronds exhibit slightly lower ultimate stress and Youngâs modulus values compared to EcoLeaf, despite their inherent strong mechanical properties, which can be primarily attributed to the cross-linking effect of its 3D mesh matrix. The manufacturing process of EcoLeaf leads to a more homogeneous and optimized structure, resulting in higher mechanical properties. This means that EcoLeaf is more resistant to deformation and can withstand greater forces without breaking or shattering, making it more environmentally tolerant. Mechanical advantages make EcoLeaf a promising alternative to natural leaves for various applications requiring strength and durability.

a Natural leaves from Trachycarpus fortune (Tf), Cercis chinensis bunge (CcB), Pyrus ussuriensis (Pu), Zamioculcas zamiifolia engl (ZzE), Holly, and EcoLeaf (EL) respectively; b Stress-strain curves and c Youngâs modulus of natural leaves from different sources and EcoLeaf (EL 1 thickness 0.19âmm and density 0.60âg/cm3, EL 2 thickness 0.22âmm and density 0.61âg/cm3) (Parallel experiments with three sets of identical samples, data are presented as mean values +/âSEM).
The efficiency of light reaction, a pivotal process in plant photosynthesis, is influenced by factors like chlorophyll species and content, chloroplast structure, and the catalytic activity of photosynthetic enzymes. Among these, photosynthetic enzyme activity plays a crucial role in a plantâs overall photosynthetic rate. This enzyme activity is highly temperature-dependent. When the temperature changes, the protein structure and vibration frequency of CA will change to different degrees, which will lead to changes in enzyme protein activity. In the natural environment, natural leaves can sustain their enzyme-based carbon sequestration capacity without requiring additional energy sources, utilizing natural light (0â1 Ã105 lux). Ecoleaf, much like natural leaves, has enzyme activity that is temperature-sensitive. As a result, this study employed spray staining (Supplementary Fig. 3a, b) to endow artificial leaves with a photosynthesis-like effect similar to that of their natural counterparts. This approach allows CA activity to be maintained by heat energy generated from light (0â1 Ã105 lux) under varying environmental conditions, without the use of an external heat source, in line with the natural photosynthesis process. As observed in Fig. 3a, b, when the leafâs ambient temperature was set at 30â°C, the initial warming rate and final temperature of the EcoLeaf increased as the K/S value increased, while the light level remained constant. Notably, the final temperature reached 55â°C when exposed to 9.4 Ã104 lux light, surpassing the optimal carbon sequestration temperature of EcoLeaf (40â°C, Supplementary Fig. 3c). For practical CO2 capture applications, the Ecoleaf variant with the most efficient photothermal conversion should be deployed in natural environments to achieve optimal carbon capture. In conditions of high visible light intensity, light avoidance methods (similar to greenhouses) can be employed to ensure CAâs optimal carbon capture activity. Conversely, under low ambient temperature or weak light intensity, the light utilization efficiency of EcoLeaf can be adjusted to meet CAâs optimal carbon capture temperature. This is achieved by either increasing the K/S value or incorporating materials with enhanced light-heat conversion capacity. This adaptability is essential for the broader climate applicability of artificial leaves. In this study, enzyme activities were also explored for two groups of EcoLeaf with different K/S values exposed to the same light level (Supplementary Fig. 3(d)). Under an initial temperature of 30â°C and after 3âmin of irradiation with a light level of 4.5 Ã104 Lux, enzyme activities for EcoLeaf with K/S values of 3.5 and 86.7 increased by 135%. This highlights EcoLeafâs capability to maintain its carbon sequestration features solely using visible light as an energy source, akin to real leaves, a critical aspect for sustainable development.

Effects of K/S value and visible illuminance on (a) initial warming rate and (b) final arrival temperature of artificial leaves at an initial temperature of 30â°C.
Light plays a pivotal role in governing the expansion and contraction of stomata on natural leaves. During daylight, in the presence of visible light, leaves tend to expand stomata to facilitate the transport of CO2, oxygen, and water vapor, whereas at night, in darkness, they close stomata. This adaptive stomatal behavior is crucial for optimizing the cycle of photosynthesis and respiration. Drawing inspiration from this natural mechanism, we synthesized GMA/4,4â-AZO from GMA and 4,4â-AZO, capitalizing on azobenzene moleculesâ unique photoisomerization property, as depicted in Fig. 4a. Employing this principle, we grafted GMA/4,4â-AZO as a monomer to form a three-dimensional network matrix on the blade surface. When exposed to various light sources, the matrixâs mesh size cyclically and reversibly changes in response to the monomersâ spatial orientation, thereby controlling the stomata of the artificial bladeâs expansion and contraction. Firstly, the stomata of EcoLeaf were visualized by high-resolution TEM in this study. As shown in Supplementary Fig. 4, with the increase in TEM magnification, the stomatal structure distributed on the 3D mesh mechanism can be clearly seen, and the stomatal size is only in the nanometer scale (0â2ânm). In addition, as illustrated in Fig. 4b, c, irradiation of EcoLeaf with a 450ânm visible light source (19.1âmW/cm2) and a 365ânm ultraviolet light source (20.3âmW/cm2) caused the absorption peaks of trans 4,4â-AZO at 390ânm to decrease and increase with irradiation time, respectively. This observation confirms that GMA/4,4â-AZO retains its photoresponsive properties, as shown in Supplementary Fig. 5, even after successful grafting onto the fiber-based material. The isomerization of GMA/4,4â-AZO leads to corresponding changes in the matrixâs mesh size and pore volume, forming the foundation for light-driven pore size regulation. As depicted in Fig. 4d and Supplementary Fig. 6, one round of irradiation (450ânm, 365ânm, and 450ânm) resulted in decreased and increased then pore volume, surface aera and average pore size of EcoLeaf. This demonstrates that light excitation at different wavelengths can directionally regulate EcoLeafâs pore size characteristics, which, in turn, controls the CO2 capture and the transport of H2O and carbon sequestration products. The pore size data further validate the range of pore sizes observed by high-resolution TEM. Additionally, we examined the variations in the optical contact angle of the EcoLeaf surface under cyclic excitation with different light wavelengths. As shown in Fig. 4e, light excitation at 450ânm decreased the water contact angle, while 365ânm excitation increased it. This change is primarily attributed to the alteration in stomatal aperture on the EcoLeaf surface. When irradiated with 450ânm light, the pore volume and average pore size increased, causing the water contact angle to decrease and vice versa. Thus, the light-responsive properties of the leafâs surface provide it with an adjustable stomatal structure. In practice, natural sunlight contains both 365ânm UV and 450ânm blue light. Therefore, the stomatal expansion and contraction of EcoLeaf under sunlight is critical. As shown in Supplementary Fig. 7, when exposed to natural sunlight containing both 450ânm and 365ânm, the EcoLeaf irradiated with the 365ânm wavelength is more inclined to absorb the blue light of 450ânm, which puts the stomata in a dilated state. Detailed analysis is located under Supplementary Fig. 7.

a Schematic diagrams of the changes in the molecular structure of GMA/4,4â-AZO and the stomata of EcoLeaf under different wavelengths of excitation light; photoresponse properties of EcoLeaf under (b) 365ânm UV and (c) 450ânm visible excitation; d Changes in the pore volume, average pore size and surface area of EcoLeaf under 450ânm visible and 365ânm UV excitation; e Changes in the optical contact angle of EcoLeaf under 450ânm visible and 365ânm UV excitation (Parallel experiments with three sets of identical samples, data are presented as mean values +/âSEM); Effects of stomatal expansion and contraction characteristics on the (f) temperature stability and (g) pH stability of EcoLeaf.
To explore the impact of stomatal expansion and contraction characteristics on carbonic anhydrase (CA), we measured CAâs acid resistance, and high-temperature resistance using the esterase method before and after light excitation at different wavelengths. As shown in Fig. 4f, g, CA exhibits better acid resistance and high-temperature resistance in the contracted-stomata state compared to the expanded-stomata state. This observation suggests that when the external conditions are suitable, expanded stomata enhance CO2 transmission, increasing carbon sequestration per unit area. Conversely, when the stomata are constracted, the denser matrix mesh insulates CA from external substances, preserving enzyme activity and maintaining protein conformation35,36. This isolation is beneficial for resisting the challenges of natural environments, extending EcoLeafâs service life, and securing its functionality. In addition, during natural carbon sequestration, CO2 enters the leaf body when the stomata are expanded. After the leaf stomata are constracted, CO2 can be converted to high-value products through a stable dark reaction. Similar to this process, the stomatal expansion and contraction characteristics of EcoLeaf can sequester carbon when the stomata are expanded, and after the stomata are constracted, CO2 can be converted into high-value products through stable and continuous catalytic conversion of CO2 by a multi-enzyme cascade reaction.
The effect of stomatal expansion and contraction characteristics on the carbon capture performance of EcoLeaf was investigated in this study by the device shown in Fig. 5a. As depicted in Fig. 5b, the CO2 capture rate of EcoLeaf was markedly reduced when exposed to 365ânm UV illumination, in contrast to the 450ânm. These changes can be attributed to the expansion and contraction of stomata. Expanded stomata offer larger pore sizes, surface area, and pore volume, facilitating substrate transportation to CA, EcoLeafâs primary carbon-sequestering component. Conversely, when the stomata contract, the leafâs pore size decreases, limiting the contact between CO2 and CA to some extent and interrupting CO2 sequestration. This control mechanism fine-tunes the CO2 capture process by EcoLeaf. In addition, analyzing from the molecular point of view, the change in exposed groups due to the change in pore structure will also affect the contact probability of EcoLeaf with CO2 to some extent. In summary, from the macroscopic changes in pore structure to the microscopic changes in the relative positions of molecules, the carbon fixation rate during stomatal contraction can be adequately explained to be lower than that during stomatal expansion.

a Schematic diagram of the CO2 capture device; b Effect of stomatal expansion and contraction states on the carbon capture performance of EcoLeaf (Parallel experiments with three sets of identical samples, data are presented as mean values +/âSEM); c Litmus color diagrams of EcoLeaf embedded with litmus test solution after CO2 capture and litmus color diagrams of EcoLeaf after placing its petiole in deionized aqueous solution for transporting H2CO3 at a room temperature of 30â°C; d Changes in water content of EcoLeaf with and without water supply (365ânm and 450ânm) (Parallel experiments with three sets of identical samples); e Long-term carbon sequestration stability of EcoLeaf with and without water supply and cycling stability of EcoLeaf with water supply.
The essence of photosynthesis in natural leaves lies in the process of carbon assimilation by the photosystem utilizing light energy. This process results in the synthesis of glucose from CO2 and water, ultimately leading to the formation of starch stores37,38. In addition, water and carbon sequestration products share transport pathways. Water is primarily transported from the petiole to various parts of the plant body through conduits, ultimately reaching the leaves, where it is utilized39,40. Carbon fixation products are transported through conduits to different parts of the plant, such as roots, stems, flowers, and fruits, to facilitate cell growth. This efficient capture of CO2 by CA relies on the participation of H2O. Additionally, if the carbonic acid produced by CA, after capturing CO2, is retained within the artificial leaf, it can lower the pH in CAâs vicinity, leading to the dissociation of enzyme subunits and affecting CAâs CO2 capture capacity. Hence, adequate water and carbon fixation product transport is crucial in EcoLeaf to maintain the activity of photosynthetic enzymes and prevent product inhibition. The structural properties of cellulose paper fibers are similar to those of natural leaf veins, allowing solutions to spontaneously diffuse and transport within them due to osmotic pressure. In this study, we examined the water-carbon sequestration product transport properties of EcoLeaf by immersing leaf petioles in branches with ample water content (Fig. 1a). As evident in Fig. 5c, EcoLeaf, embedded with litmus, rapidly turned red upon absorbing gaseous CO2. However, after placing its petiole in water for 20âmin, the red litmus solution reverted to its original blue-violet color. This transformation occurs because the production of H2CO3 (carbonic acid) within EcoLeaf, following CO2 capture, creates an acidic microenvironment, causing the litmus test solution to turn red. When the leaf petiole is immersed in water, H2CO3 within the EcoLeaf matrix migrates through the leafâs fibers to the aqueous solution, reducing the H2CO3 concentration and restoring the matrixâs microenvironment to its initial state, turning the litmus test solution blue-purple again. Therefore, EcoLeafâs natural fiber mesh structure can serve as a conduit similar to those in natural leaves, facilitating the transport of water and CO2 sequestration products. This capability opens the door to long-term cyclic carbon sequestration.
Artificial leaves, when exposed to the external environment for extended periods for gaseous CO2 capture, experience surface water evaporation. This results in the loss of the proton source required for CA in capturing gaseous CO2. Thus, timely replenishment of water is crucial. As illustrated in Fig. 5d, without a petiole connected to a water source, the water content in the EcoLeaf progressively diminishes over time, leading to water dissipation. Conversely, when the petiole is inserted into a watery branch, the leafâs water content remains relatively stable over time. This indicates that the water source within the branch continuously supplies water to the CA in the umbrella area under the influence of osmotic pressure. While the water content of the leaf blade remains constant, water and carbonic acid molecules are in constant motion. To further explore the impact of stomatal expansion and contraction characteristics on leaf water cycling properties, this study was conducted to examine the variations in water content of EcoLeaf under 365ânm and 450ânm light excitation, respectively. As shown in Fig. 5d, When the roots of EcoLeaf were in contact with a water source and the stomata were contracted (365ânm), a decrease in water content was observed compared to when the stomata were expanded (450ânm), indicating that the expansion and contraction states of the stomata have an impact on the water transport properties of EcoLeaf. With expanding stomata, driven by the osmotic pressure of the liquid, water molecules were more likely to pass through the pores of the 3D mesh fabric matrix to reach the leaf surface, providing an adequate source of protons for CA carbon sequestration. However, when the stomata of EcoLeaf were contracted, a reduction in its own water content occurred, indicating a weakened rate of water transport. This weakening was primarily due to the stomatal contraction, which increased the density of the three-dimensional mesh fabric matrix of EcoLeaf and opposed the liquid osmotic pressure, hindering the transport of water molecules. This finding further supports the conclusion in the Fig. 5b. Furthermore, when there was no water supply, the rate of water loss (k2) of EcoLeaf in the pore-contracting group (365ânm) was slightly higher than that of the pore-expanding group (450ânm, k1) in the primary stage. This may due to the contraction process of the stomata induced by the UV light at 365ânm, which squeezed some of the water outside the EcoLeaf. Importantly, the decreasing rate of k2 value was much lower than that of k1. This is mainly attributed to the water retention effect caused by the contraction of the mesh, which prevents the volatilization of water molecules retained inside the mesh. As a result, the water content of the contracted group has become higher than the expanded group in the later stage. In conclusion, EcoLeaf can achieve a dynamic equilibrium, facilitating the water-carbonic acid transport process. This prevents the adverse effects of leaf dehydration and product inhibition on the carbon sequestration capacity of artificial leaves. Consequently, EcoLeaf can consistently deliver its performance in capturing gaseous CO2.
The long-term carbon sequestration stability of EcoLeaf is shown in Fig. 5e, CO2 capture rate of EcoLeaf with water supply is significantly higher than that of EcoLeaf without water supply. This is mainly attributed to the greater proton availability in EcoLeaf with higher water content. This enhanced proton supply enhances CAâs efficiency in converting CO2, resulting in an overall improvement in EcoLeafâs carbon sequestration efficiency. The CO2 capture ability of EcoLeaf with water supply also gradually stabilized over the long-term and cycling tests without significant product inhibition. In addition, the second and third batch sequestration rates were essentially unchanged over time, thus demonstrating that the sequestration behavior of EcoLeaf tends to be in transport equilibrium and does not result in inhibition of product accumulation. This is mainly attributed to its substance-transport properties. In addition, the CO2 capture ability of EcoLeaf without water supply was unstable and gradually weakened due to the inhibition of products and proton sources. This further demonstrates that the material transport property enables the EcoLeaf prepared in this study to maintain a stable carbon sequestration capacity over a long period of time.
In order to investigate whether CA, the core organ of carbon sequestration, would undergo enzymatic leakage during H2O-H2CO3 transport, the aqueous solution during transport was qualitatively and quantitatively analyzed by LSCM and Caumas Brilliant Blue staining (G250) in this study. As shown in Supplementary Fig. 8a, within 0â50âmin after the petiole of EcoLeaf containing FITC-CA was connected to the water source, the aqueous solution contained only a very small amount of weak green fluorescent dots and did not show bright green fluorescence. This provides preliminary evidence that no leakage of CA occurred. In addition, Supplementary Fig. 8b shows that CA leakage rates remain extremely low, which further proves that no obvious enzyme leakage occurred in EcoLeaf. Additionally, in this study, the carbon capture core organ CA was replaced with formate dehydrogenase (FDH) to verify the designability of the EcoLeaf bionic system. As shown in Supplementary Fig. 9, CO2 can be successfully converted to HCOOH by replacing CA with FDH and NADH in the EcoLeaf bionic system. This demonstrates the high degree of designability of the EcoLeaf system.
In order to further explore the practical application value of EcoLeaf, we have summarized emerging CO2 conversion systems with high light energy conversion efficiency and analyzed material cost, preparation method, light utilization efficiency, and potential environmental impact (Supplementary Table 1). Compared to existing light source-driven CO2 treatment systems, EcoLeaf has low-cost raw materials, mild preparation conditions, and is able to be degraded by natural soil and participate in further ecological cycles. The efficiency and cost of light energy utilization are decisive for the large-scale preparation and application of materials. Therefore, this study quantifies the prospect of large-scale application (valuation of large-scale application) of the materials through the efficiency/cost of light energy utilization. Supplementary Fig. 10 shows that the valuation of scale-up applications of EcoLeaf achieved 4.9âmol·Jâ1·$â1 in the first place compared to existing carbon sequestration materials. It can be seen that EcoLeaf has significant advantages over existing carbon sequestration strategies after quantifying the economic costs and energy efficiency. Thus, the design and preparation of EcoLeaf provide a sustainable and effective way to achieve the goal of carbon neutrality.
Natural leaves ultimately wither and become part of the ecosystemâs nutrient-cycling process. During this phase, leaves gradually degrade, releasing various elements that return to the soil. This nutrient source becomes available for the plant root system to absorb. However, the existing artificial leaf system cannot be degraded by the natural environment, and will face the problem of secondary pollution while sequestering carbon, which seriously limits its large-scale application. Because EcoLeafâs primary framework closely resembles the cellulose fraction of natural leaves, it can effectively mimic the natural process of leaf degradation.
As depicted in Fig. 6a, natural leaves and EcoLeaf undergo the process of returning to the soil. The figure illustrates that EcoLeaf, similar to natural leaves, fully participates in the alternation between wet and dry soil conditions and the metabolic processes involving various microorganisms and enzymes. The degradation rate of EcoLeaf is slightly higher than that of natural leaves. By day 20, 50% of EcoLeaf had degraded in natural soil, while only 32% of natural leaves had degraded. By day 40, EcoLeaf had almost completely degraded, while natural leaves remained. This is primarily due to the fact that EcoLeafâs main framework (cellulose) is more homogeneous compared to the composition of natural leaves, which include lignin, cellulose, and hemicellulose, making EcoLeaf more easily degradable.

a Soil degradation and mass loss of natural leaves and EcoLeaf at room temperature (Parallel experiments with three sets of identical samples, data are presented as mean values +/âSEM); b Soil eco-culture maps of natural leaves (A-1-A-2), EcoLeaf (B-1-B-2), and blank group (C-1-C-2) at room temperature.
To investigate whether the degradation products of EcoLeaf affect the soil microenvironment, we used the degraded soil to cultivate canola crops. As Fig. 6b demonstrates, soil from both EcoLeaf degradation and natural leaf degradation, as well as the canola seedlings planted in the control group, showed similar growth patterns. This is because the degradation products of both natural leaves and EcoLeaf consist of essential soil elements (C, H, O, and N) required for plant growth, and they do not disrupt the soil microenvironment. This indicates that EcoLeaf can smoothly participate in the ecosystemâs nutrient cycling process, akin to natural leaves, illustrating the significance of the ancient Chinese poem, Turning into spring mud protects the flowers.
In this study, we successfully developed EcoLeaf, a biomimetic construct closely resembling natural leaves. The mechanical properties of EcoLeaf are higher than those of most natural leaves but are similar to those of palm leaves, making it less prone to pulverization and providing excellent environmental resistance. The EcoLeaf can convert visible light energy into controlled thermal energy to maintain the optimal activity of CA for carbon capture. This control is achieved by regulating the depth of leaf staining and light intensity. Through cyclic excitation using 365ânm and 450ânm light sources, the stomata within the EcoLeaf substrate cyclically contract and expand, allowing for the controlled switch between self-protection in harsh environments and high-efficiency carbon capture in suitable conditions. The connection of the petiole to a water source ensures a steady proton supply for capturing gaseous CO2 while preventing the accumulation of carbon sequestration products. Soil degradation experiments indicate that EcoLeaf can completely degrade in natural soil within 40 days, and the resulting degradation products have no adverse effects on the soil microenvironment. This ensures a stable ecological cycle. Compared to existing synthetic blades, EcoLeaf offers the best efficiency/cost ratio and serves as a biomimetic and functionalized platform for artificial biocarbon sequestration. Moreover, its biocarbon sequestration pathway exhibits a certain degree of design flexibility, allowing for adaptation to various single-enzymatic or multi-enzymatic cascade catalysts for converting gaseous CO2 into more abundant C2/long-chain products. The EcoLeaf represents a significant step towards the development of enzymatic artificial leaves.