Dissipative chemical processes, due to their potential for far-from-equilibrium properties and functions, have attracted significant attention in the development of lifelike materials and systems. These processes transform energy from one form to another.
In recent years, chemists have sought to harness these processes to design systems with specific functions. However, achieving mechanical functions through dissipative self-assembly, similar to those found in biological structures like microtubules, remains a challenge.
In a study published in Nature Chemistry, Prof. Liu Kai from the Shenzhen Institutes of Advanced Technology (SIAT) of the Chinese Academy of Sciences, collaborating with a team led by Prof. Sijbren Otto from the University of Groningen, has developed an active droplet system that can exhibit chemotactic movement through the cross-scale conversion of energy.
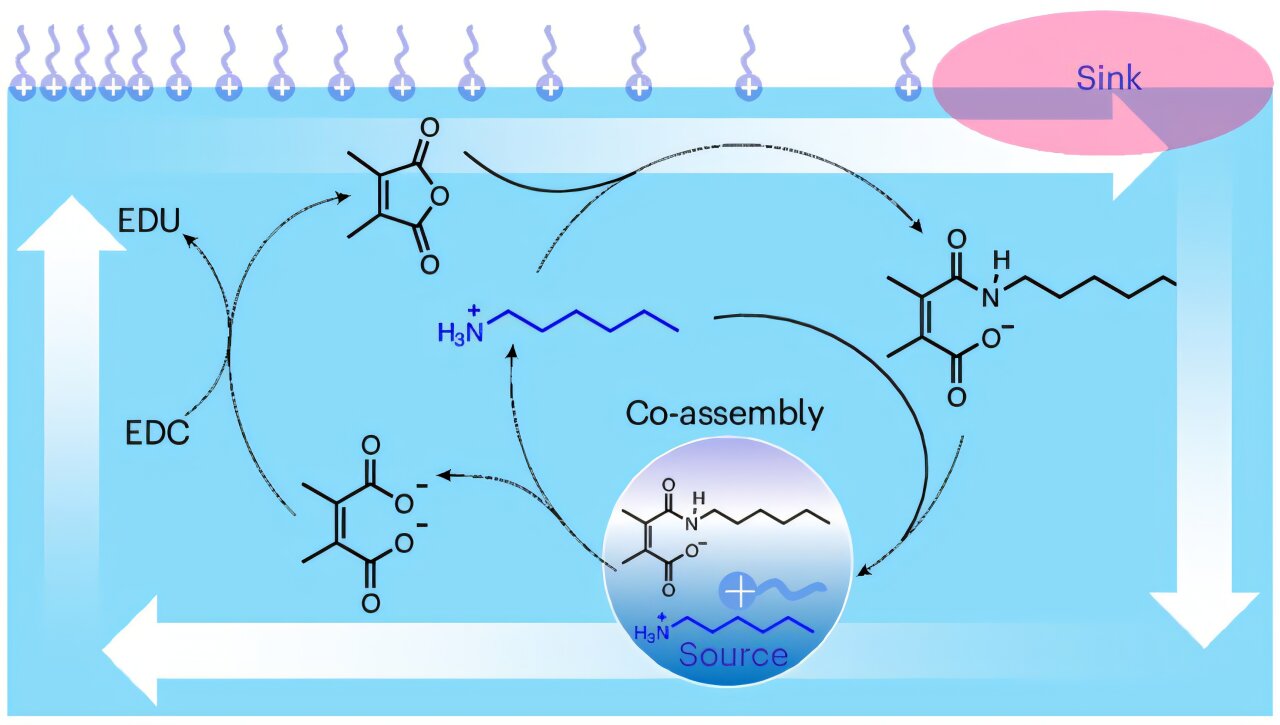
Researchers created the active droplets by employing a dissipative reaction network that involves a dynamic amide bond formed by mixing maleic anhydride and octylamine in an aqueous solution. This bond was also readily hydrolyzed under acidic conditions. Carbodiimide served as a secondary fuel molecule, driving the reformation of the amide compound from the diacid waste and octylamine, thus establishing a dissipative cycle.
The amide product and octylamine co-assembled into droplets through intermolecular electrostatic and hydrophobic interactions. The dynamics of this self-assembly could be tuned by the addition of chemical fuels, resulting in transient growth of the droplets.
When oleic acid was added to the surface of the droplet solution, the droplets on the air-water surface moved towards the oleic acid. This behavior was resulting from the coupling between the dissipative self-assembly process and the Marangoni effect, which induced Marangoni flow that propels the movement of the droplets. In addition, the speed and duration of the droplets’ motion could be precisely regulated.
In this system, chemical fuels drive the formation of amide bonds at the molecular level, while at the nanoscale, they promote the creation of high-energy active droplets. On the macroscopic scale, these fuels enable the directed movement of the droplets.
“Our study demonstrates that high-energy dissipative assemblies can function as energy converters, which may bring new perspectives to the development of active materials, such as micro-nano motors, soft robots and non-equilibrium patterns,” said Prof. Liu. “The proposed droplet system, composed of simple molecules, could serve as a model for primitive cells, capable of exhibiting complex collective behaviors.”
More information:
Kai Liu et al, Molecular-scale dissipative chemistry drives the formation of nanoscale assemblies and their macroscale transport, Nature Chemistry (2024). DOI: 10.1038/s41557-024-01665-z
Provided by
Chinese Academy of Sciences
Citation:
Chemists develop dissipative droplet system capable of chemotactic movement (2024, November 14)
retrieved 16 November 2024
from https://phys.org/news/2024-11-chemists-dissipative-droplet-capable-chemotactic.html
This document is subject to copyright. Apart from any fair dealing for the purpose of private study or research, no
part may be reproduced without the written permission. The content is provided for information purposes only.