Design of a protein ligase based on the structure of OaAEP1-C247A
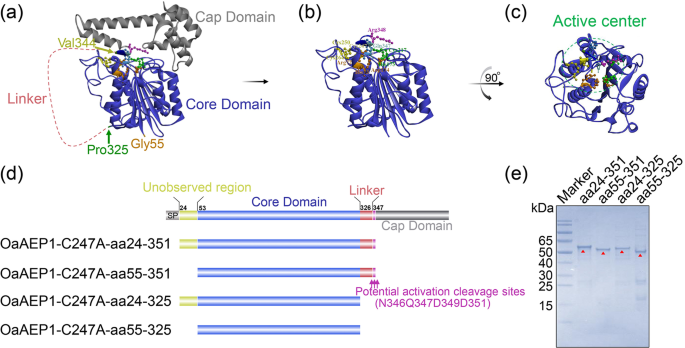
According to the structure basis, the 271 amino acid long core domain of OaAEP1 is essential to exert its ligation activity (Fig. 1a). But the catalytic cleft is entirely shielded by the cap domain (Fig. 1a). So, the release of cap domain is greatly warranted for protein or peptide substrates to get access to the active site (Fig. 1b, c). Currently, it remains unclear whether aa24-54 and aa326-351 play crucial roles in the ligase function. Therefore, four truncations were designed: OaAEP1-C247A-aa24-351, OaAEP1-C247A-aa55-351, OaAEP1-C247A-aa24-325, OaAEP1-C247A-aa55-325 (Fig. 1d, nucleotide sequences of the four truncations see Supplementary Methods in the Supplementary Information). These truncations were expressed in E. coli as fusion proteins with N-terminal TrxA-Tag and C-terminal His-tag and were purified using a cobalt-based IMAC column (Supplementary Fig. 1a, b). The proteins with high levels of purity were obtained and confirmed by SDSâPAGE analysis (Fig. 1e and Supplementary Fig. 1c). Taking the molecular weight of the fusion protein TrxA, his-tag, linker, and that of the target protein into account, the size of each recombinant OaAEP1-C247A truncations agrees with the expected molecular weight: OaAEP1-C247A-aa24â351, 55âkDa; OaAEP1-C247A-aa55-351, 51âkDa; OaAEP1-C247A-aa24-325, 52âkDa; OaAEP1-C247A-aa55-325, 48âkDa.
a Crystal structure of OaAEP1 as zymogenic form (from PDB code: 5H0I). b Active form of OaAEP1 by removal of C-terminal cap domain. c The top view of activated OaAEP1 is obtained by 90° rotation around the vertical as presented in (b). S1 pocket (Active center) with active site residues displayed as scaled balls, sticks, and labeled. d Schematic representation of the truncated activated OaAEP1. SP signal peptide; Unobserved region means the region unobserved in crystal structure. e SDSâPAGE analysis of fractions (1âμg) after purification of the OaAEP1-C247A truncations. Red triangles indicate the target bands.
Ligation activity of active OaAEP1
In order to evaluate the ligation activity of the four truncated proteins, we synthesized the model substrate Pep133-NGL. This substrate was created by adding the recognition motif of OaAEP1 ligase âAsn-Gly-Leuâ to the C-terminal of peptide aa133-147 of human cytomegalovirus pp65 protein. Another peptide having N-terminal residues, âGly-Leuâ (GL- (biotin-labeled) peptide), was utilized as the nucleophile. The enzymatic conversion product was analyzed via enzyme-linked immunosorbent assay (ELISA) using an antibody against peptide aa133-147 (Fig. 2a). All four truncated proteins exhibited enzymatic ligating activity, with OaAEP1-C247A-aa55-351 performing the best (Fig. 2b, Supplementary Data 2). Therefore, OaAEP1-C247A-aa55-351 was further investigated.
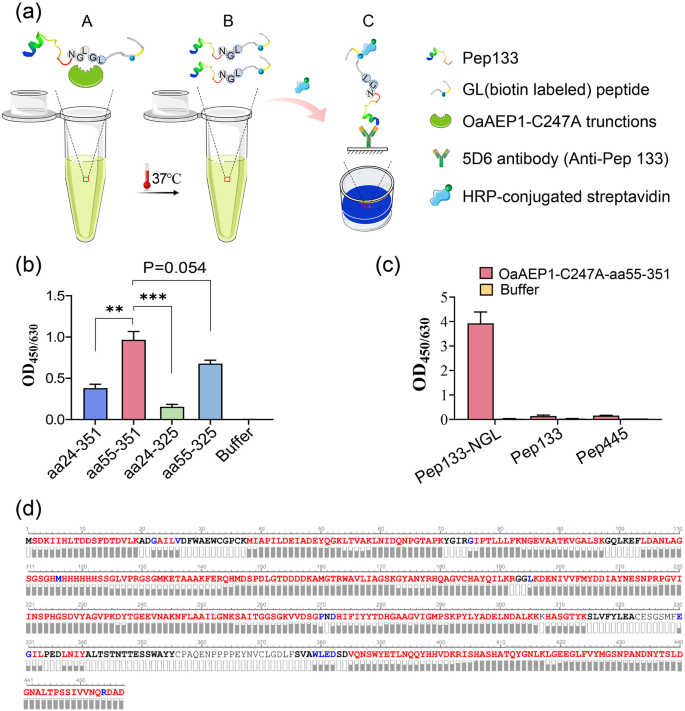
a Schematic representation of ligating assay and the evaluation mode of the reaction products after ligating by the truncated OaAEP1. âNGLâ, âGLâ based peptides and truncated OaAEP1 mixed at proper buffer (A) and ligating at 37â°C. Ligating products (B) were subjected to capture by antibodies against âNGLâ based peptide and detected by HRP-conjugated streptavidin (C). b The ligating activity of the different truncated OaAEP1-C247A. Pep133-NGL (3âμM) and GL-(biotin-labeled) peptide (30âμM) were ligated by the different truncated OaAEP1-C247A (0.5âμM) at 37â°C in PBS for 30âmin, and the ligating products were detected by ELISA showed in (a). c The specificity of OaAEP1-C247A-aa55â351 was verified by peptides of CMV pp65 free of Asn-Gly-Leu. Pep133-NGL/Pep133/Pep445: 5âμM; GL-(biotin-labeled) peptide: 50âμM; OaAEP1-C247A-aa55â351: 1.5âμM. Multiple groups were compared using ordinary one-way ANOVA. **pâ<â0.01, ***pâ<â0.001. d Sequence coverage view of OaAEP1-C247A-aa55-351 protein by LCâMS/MS analysis on an Orbitrap Eclipse Tribrid mass spectrometer. The amino acids are colored in red, blue and black, which represents three different levels of amino acid confidence from high to low. The assays are performed as nâ=â3 biologically independent experiments, and the bars represent the mean and standard deviation (SD).
To elucidate whether only peptide containing the reported recognition site âAsn-Gly-Leuâ can be recognized and ligated by OaAEP1-C247A-aa55-351, other peptides of pp65, Pep445, and Pep133 were used to replace Pep133-NGL as substrate, and the results showed that none of these peptides could be ligated with the biotinylated GL peptide (Fig. 2c, Supplementary Data 2), which illustrates the substrate specificity of OaAEP1-C247A-aa55-351.
To determine whether auto-cleavage occurred during the expression and purification of OaAEP1-C247A-aa55-351, amino acid sequencing was performed to determine its C-terminal boundary. Purified OaAEP1-C247A-aa55-351 proteins were separated by SDSâPAGE, followed by in-gel digestion with trypsin, and subjected to amino acid sequencing by LC-MS/MS. The theoretical amino acid sequence of the recombinant OaAEP1-C247A-aa55-351 (Supplementary Fig. 1a) was used as a template to obtain the sequence. Auto cleavage was not observed according to the result of LCâMS/MS. The amino acid sequence was consistent with the theoretical sequence (Fig. 2d).
Optimization of the ligating conditions of OaAEP1-C247A-aa55-351
The ligating efficiency may be greatly affected by the buffer conditions, specifically the pH and the presence of metal ions. Previous studies have reported that OaAEP1-C247A displays high catalytic activity towards P1-Asn substrates at a neutral pH level (pH 7.0)40. To determine the pH preference of OaAEP1-C247A-aa55-351, pH scanning assays were conducted in various pH buffers ranging from pH 4.0 to 9.6 (Fig. 3a). The ligating products were tested using ELISA as previously described in Fig. 2a. The results showed that OaAEP1-C247A-aa55-351 displayed ligation activity between pH 4.0 and 8.0, with the highest ligating efficiency observed between pH 7.0 and 7.2 (Fig. 3b, Supplementary Data 2). The enzyme exhibited reduced activity in acidic or basic buffer conditions, but certain activity was observed at pH 4.0 and 8.0 (Fig. 3b, Supplementary Data 2). These findings suggest that the pH preference of OaAEP1-C247A-aa55-351 is similar to the original OaAEP1-C247A.
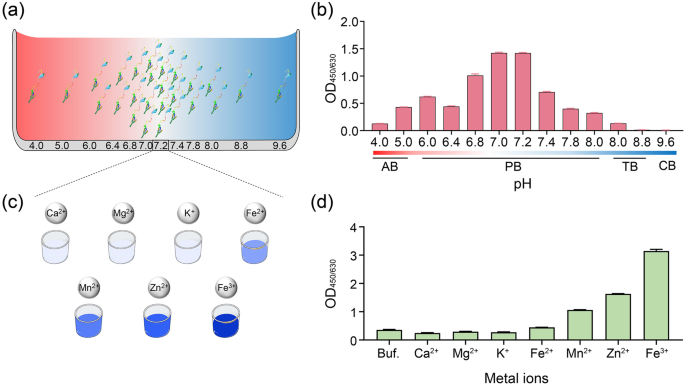
Schematic diagram of ligating substrates and nucleophiles under buffer different pH (a) and metal ions (c). AB: 50âmM sodium acetate buffer; PB: 20âmM phosphate buffer; TB: 50âmM TrisâHCl buffer. b The ligating activity of Pep133-NGL (2âμM) and GL-(biotin-labeled) peptide (10âμM), which ligated in buffers with different pH by the OaAEP1-C247A-aa55-351 ligase (0.5âμM) at 37â°C for 30âmin. d The effect of different metal ions (100âμM) in enhancing the ligating efficiency of OaAEP1-C247A-aa55-351. Buf.: Buffer (phosphate buffer, pH 7.2); Ca2+: CaCl2; Mg2+: MgCl2; K+: KCl; Fe2+: FeSO4; Mn2+: MnCl2; Zn2+: ZnSO4; Fe3+: FeCl3. The assays are performed as nâ=â3 biologically independent experiments, and the bars represent the mean and SD.
Metal ion, such as Mg2+, Mn2+, Ca2+, Zn2+, Fe3+, can facilitate various enzymatic reactions49,50,51. Considering that no available studies have examined the role of metal ions on the ligase activity of OaAEP1, we tested several common metal ions, including Ca2+, Mg2+, K+, Fe2+, Fe3+, Mn2+, Zn2+ (Fig. 3c). The results demonstrated that the first three metal ions had no significant effect on the ligation activity of OaAEP1-C247A-aa55-351, whereas the other four ions facilitate the ligating activity of OaAEP1-C247A-aa55-351, among which Fe3+ performs the best (Fig. 3d, Supplementary Data 2).
Substrate and nucleophile specificity of OaAEP1-C247A-aa55-351
Previous studies have reported the C-terminal tripeptide recognition motif of OaAEP1, Asn-Gly-Leu (NGL, P1âP1ââP2â) is broken between P1 and P1â during the catalytic process. This occurs as the catalytic cysteine of OaAEP1 performs a nucleophilic attack and leads to the formation of the acyl-enzyme intermediate between the catalytic cysteine and the P1 Asn residue34,37,40,43,52. Then, the amine group of the N-terminal Gly-Leu (GL)-based nucleophile peptide (P1ââP2â) attack onto the formed unstable acyl-enzyme intermediate breaks the transient thioester bond and releases the ligating product from the catalytic cysteine37,43,52. Previous studies have demonstrated the recognized and nucleophilic motif for peptide ligation by OaAEP1, but the results were not so consistent and adequate53,54. To further validate and augment the profiles of recognition and nucleophile peptides, five positions, namely P1âP1ââP2â and P1ââP2â, were substituted with Ala, Gln, Phe, Asp, Lys, respectively, representing amino acids categorized as non-polar aliphatic, polar neutral, aromatic, acidic and basic amino acids (Fig. 4a). In accordance with prior findings, P1 Asn exhibited relatively stringent requirements, as substitution of P1 with other amino acids resulted in the unidentifiable and unligated peptides (Fig. 4b, Supplementary Data 2). On the other hand, the positions of P1â or P2â were found to be less restricted. Interestingly, When P1â G was replaced by A or K, the ligating efficiency was further improved, with P1â A demonstrating the best performance overall (Fig. 4b, Supplementary Fig. 2b and Supplementary Data 2). Though P1â Q or F and P2â A or F remained recognizable, their ligation efficiencies were lower (Fig. 4b, Supplementary Data 2). In terms of the nucleophile peptide, P2â displayed greater stringency compared to P1â, and it exhibited a nucleophilic attack capability only when P2â L was substituted with F and not with the other four amino acids (Fig. 4c, Supplementary Data 2). While P1â exhibited more flexibility, P1â F or P1â K outperformed the original P1â G, with Pâ K showing the highest efficiency (Fig. 4c, Supplementary Data 2). Furthermore, to determine the optimal âpartnerâ, P1â A and P1â K are substituted with amino acids possessing similar characteristics and subsequently paired (Fig. 4d), and the combination of Asn-Ala-Leu (NAL, P1âP1ââP2â) with Arg-Leu (RL, P1ââP2â) achieved the highest ligation efficiency (Fig. 4e, Supplementary Fig. 2c and Supplementary Data 2). Moreover, the presence of position P1â at the N-terminus of the peptides or proteins was essential for successful ligation (Fig. 4c, Supplementary Data 2). The superior ligating activity of Asn-Ala-Leu (NAL, P1âP1ââP2â) paired with Arg-Leu (RL, P1ââP2â) could be attributed to two factors. Firstly, it may be attributed to the stronger electrophilic nature of Ala in comparison to Gly, making it more susceptible to nucleophilic attack. Secondly, Arg exhibits a stronger electropositive cavity than Gly, facilitating nucleophilic attack.
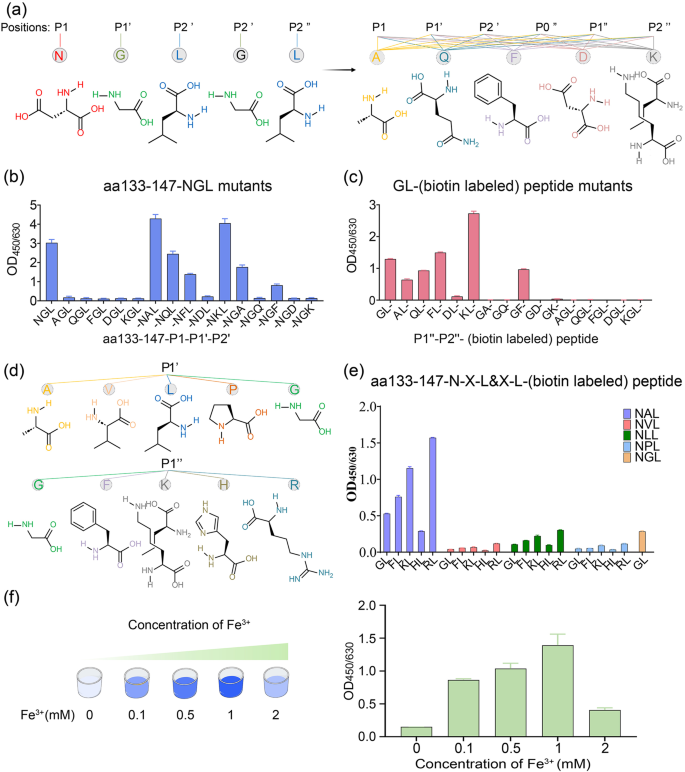
a Schematic representation of amino acid structures of the five positions of P1âP1ââP2â and P1ââP2â, and five kinds of representative amino acid including non-polar aliphatic, polar neutral, aromatic, acidic and basic amino acids. b, c The ligating efficiency of different Pep133-P1âP1ââP2â mutants to GL- (biotin-labeled) peptide (b) or Pep133-NAL to different P1ââP2ââ (biotin-labeled) peptide mutants (c). P0â indicates the position in front of P1â. d Schematic representation of amino acid structures of the same group of position P1â Ala and P1â Lys, i.e., non-polar aliphatic (Val, Leu, Pro) and basic amino acids (His, Arg). e The best partner of hydrolysis and nucleophile peptide of OaAEP1-C247A-aa55â351 (Pep133-NXL links with *L- (biotin-labeled) peptide (Xâ=âA or V or L or P or G, *G or F or K or H or R)). f The impact of concentrations of Fe3+ on the ligation activity of OaAEP1-C247A-aa55-351. The assays are performed as nâ=â3 biologically independent experiments, and the bars represent the mean and SD.
Based on the optimal substrate of Asn-Ala-Leu (P1âP1ââP2â) and nucleophile peptide of Arg-Leu (P1ââP2â), the effect of Fe3+ was further validated, and the optimum concentration of Fe3+ was investigated. The result demonstrated that a moderate concentration of Fe3+ enhances the yield of the ligating product, with 1âmM proving to be the most effective (Fig. 4f, Supplementary Data 2). Given that the catalytic residue is cystine, we introduced oxi-reductive addictives to examine the oxidation-reduction effects of metal ions on enhancing the ligase activity. However, both the oxidizing agent (H2O2) and the reducing agent (β-mercaptoethanol) appeared to have a detrimental effect on the ligation activity of OaAEP1-C247A-aa55â351, and the former seemed worse (Supplementary Fig. 2d). Therefore, the role of metal ions necessitates further exploration for a comprehensive understanding in future studies.
OaAEP1-C247A-aa55-351 mediated highly efficient peptide and protein labeling
To investigate the kinetics of OaAEP1-C247A-aa55-351 in the ligating substrate and nucleophiles, a Förster resonance energy transfer (FRET) ligation assay was conducted. This assay utilized the pep133-NXL (Xâ=âAla or Gly, FAM labeled) peptide as the substrate and the XL peptide (Xâ=âArg or Gly, Dabcyl labeled) as the nucleophile, enabling real-time monitoring of ligated product formation (Fig. 5a, b). As the substrate and the nucleophile were ligated, the close physical proximity of the two fluorophores facilitated energy transfer from FAM to Dabcyl, resulting in reduced FAM (λexâ=â510ânm) fluorescence. The decrease in relative fluorescence unit (RFU) over time demonstrated successful ligation of the substrates and nucleophiles (Fig. 5c, Supplementary Data 2). The catalytic rate exhibited a rapid rise with increasing substrate concentration, reaching a plateau thereafter (Fig. 5d). Notably, the Kcat/Km value of OaAEP1-C247A-aa55-351 towards the substrate recognition motif âAsn-Ala-Leuâ and nucleophiles âArg-Leuâ under the condition of Fe3+ was approximately 24 times higher than OaAEP1-C247A-aa55-351 towards the previously reported âAsn-Gly-Leuâ and âGly-Leuâ, and 70 times higher than the ultrafast variant AEP(Cys247Ala), as well as 2 times higher than butelase-114, the most efficient ligase reported to date (Fig. 5d, e). Interestingly, OaAEP1-C247A-aa55-351 exhibited markedly enhanced catalytic activity compared to butelase-1 or AEP(Cys247Ala). This improvement can primarily be attributed to the mild purification process, which circumvents the reduction in enzyme activity associated with acidic activation, as well as the enhanced ligating yield benefiting from more efficient recognition and nucleophile motifs, as well as the incorporation of metal ions.
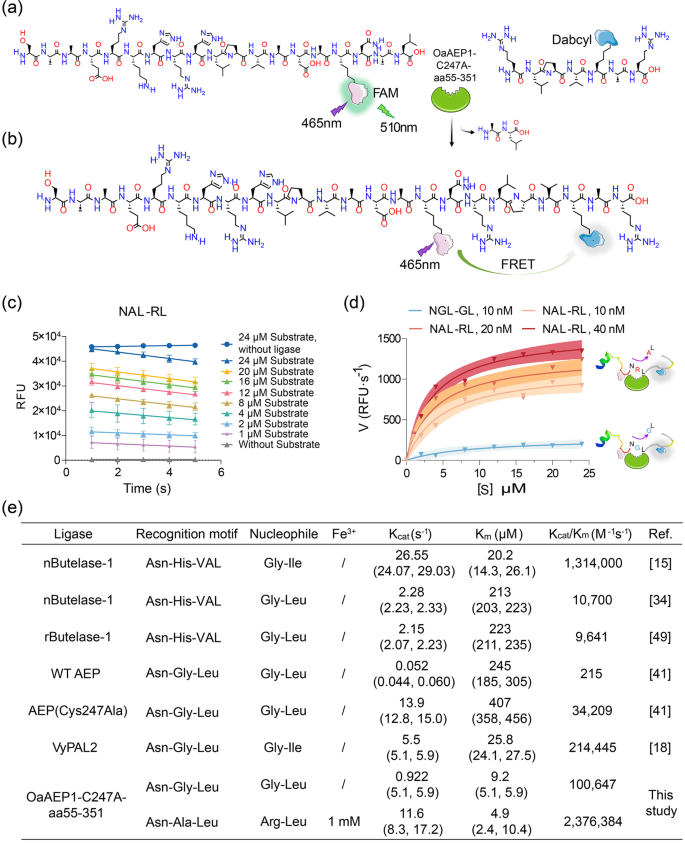
a, b Schematic representation strategy of kinetic study of OaAEP1-C247A-aa55-351 with substrate and nucleophile containing a FRET donor and acceptor, FAM and Dabcyl. Once ligation, Dabcyl comes in proximity with FAM and quenches the fluorescence of FAM emission. c Analysis of ligase activity by monitoring the RFU (FAM) value of different concentrations of substrate (âNAL-RLâ) over time with (40ânM) or without OaAEP1-C247A-aa55â351 using a FRET assay. The ligation reactions were performed with a substrate: nucleophile molar ratio of 1:3, 1âmM Fe3+ at 37â°C for 2âmin. The decline of RFU of FAM at the first 5âs is used to determine the initial velocity. The assays are performed as nâ=â3 biologically independent experiments. The data are presented as the mean, and the error bars indicate the SD. Simple linear regression was calculated and plotted in GraphPad Prism version 9.0. d The variation of catalytic rate of OaAEP1-C247A-aa55-351 with substrate concentration. The catalytic efficiency was calculated with the decreasing rate in fluorescence signal during the first 5âs after enzyme addition. The blue and red curve represents the variation of catalytic rate of OaAEP1-C247A-aa55-351 to âNGL-GLâ and new substrate and nucleophile partner âNAL-RLâ with 1âmM Fe3+, respectively. The shadow represents the error range. e Kinetic parameters for the ligation of substrates by OaAEP1-C247A-aa55-351 versus reported WT OaAEP1, Cys247Ala40, VyPAL217, and butelase-1 freshly extracted from a plant (nButelase-1)14,33 or recombinant expressed (rButelase-1)48.
To further assess the product yield following ligation by OaAEP1-C247A-aa55-351, we synthesized 50 amino acids long peptide C50-NAL and RL- (biotin-labeled) polyXXK peptide to observe noticeable changes between the ligation products and the substrates prior to ligation. After ligation by OaAEP1-C247A-aa55-351 under the buffer with Fe3+, the products were subjected to analysis by SDSâPAGE. The results indicated that over 90% of C50-NAL was ligated with RL- (biotin-labeled) polyXXK peptide (Supplementary Fig. 3a, b), demonstrating that OaAEP1-C247A-aa55-351 is capable of producing high yields of products.
Protein labeling is a powerful tool in the field of biomedical and biotechnology. To assess the ability of OaAEP1-C247A-aa55-351 to facilitate well-folded protein labeling, we expressed and purified recombinant truncated nucleocapsid protein (aa1-258) of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) with a C-terminal âAsn-Ala-Leuâ, designated as rtNP-NAL (Supplementary Fig. 3c). Nucleophiles in the form of âArg-Leuâ (RL- (biotin-labeled) peptide) were used. The resulting product was detected using ELISA, similar to Fig. 2a, utilizing the antibody against rtNP, 17H11, coated on the microplate. The results demonstrated that the biotin-labeled peptide could be conjugated to rtNP-NAL (Supplementary Fig. 3d), indicating that properly folded recombinant proteins are equally capable of site-specific modification with the assistance of OaAEP1-C247A-aa55-351.