Characteristics of the population
The median age of the participating women was 32.5 years, all of them had university education and lived in the industrial area. Most of them (nâ=â20, 67%) had a normal weight before pregnancy (BMIâ=â18.5â24.9 kg/m2), whereas 7 (23%) were overweight or obese (BMIââ¥â25.0 kg/m2). The mean infantsâ birth weight was 3573.7â±â445.7 g. The mean infantâs milk intake was 502.1â±â58.8 mL/day. Detailed data regarding maternal and infant characteristics is presented in Table 2.
Maternal dietary intake
Regarding the Polish Nutritional Standards16 the estimated average energy requirement for exclusively breastfeeding women is about 2300â2755 kcal/day. In the present study, in most of the women (nâ=â21, 70%) energy intake was below 2000 kcal and the median energy value was 1798.4 kcal/day (1524.5â2047.1 kcal/day). The main sources of the total energy value of the diet were carbohydrates (51.1â±â7.1%) and then fats (31.1â±â6.1%). Almost half of the mothers (nâ=â14, 47%) declared taking dietary supplements. Their composition was checked, and nutritional value was involved in the calculation of the maternal diet. Only 6 (20%) and 3 (10%) mothers met the recommended daily intake for calcium and zinc, respectively.
Essential and non-essential elements concentrations in human milk
In total, the concentration of 18 essential and non-essential elements was assessed in HM samples. The following elements: As, Cd, Cr, Cu, Hg, Mn, Mo, Ni, Sn, Pb, and Tl were detected in all tested milk samples (nâ=â30), whereas concentrations of Al, Ba, Be, Co, Th, U, and V were below the limit of detection of the method. Detailed information about the concentration of analyzed elements and the number and percentage of left-censored data is presented in Table 3. Considering the high proportion of left-censored results of V (90%), the descriptive statistics for this metal were not calculated. Among elements that were detected in all samples, the highest mean concentration was found for Cu (323.7 μg/L) and the lowest for Tl (0.2 μg/L).
The obtained results were compared with the usual reference levels provided by the WHO12. In all samples, Sn concentration was above the reference value of 1 μg/L. The review of the recent literature showed that data on Sn levels in HM remains scarce. A multicenter WHO study reported that Sn concentration ranged from 1.4 to 3.3 μg/L12. Although our results exceeded these values, even higher concentrations were obtained in the latest Iranian study (7.4â±â3.77 μg/L)17. Additionally, the authors17 reported that Sn content was positively correlated with maternal BMI (pâ=â0.036), in our study, we did not observe such a relationship (râ=â0.122, pâ=â0.519). Comparing the concentration of other elements with WHO levels, no exceedances were found for Cd, Hg, and Ni (Table 3).
Recently Ghane et al.5 provided a systemic review and meta-analysis to report the concentration of potentially toxic elements (As, Cd, Cu, Fe, Ni, Pb, Zn) in HM. The authors observed that the highest concentration of As (2800 μg/L) and Pb (268 μg/L) were related to the Western Pacific Region, whereas Cd (70 μg/L) to the European Region. Considering trace elements, the highest contents of Cu (1840 μg/L) and Ni (600 μg/L) were observed in the Eastern Mediterranean Region. What is important, the results of Cochraneâs Q test and I2 statistics indicate a significant heterogeneity (pâ<â0.001 for all metals) among included studies in terms of element concentrations in HM. The lowest and the highest concentrations of metals were related to Cd (150 μg/L) and Cu (1840 μg/L), respectively, which is consistent with our results. However, in our study, the obtained mean concentrations were significantly lower for both metals (0.2 μg/L and 323.7 μg/L, respectively).
Comparing our results with others which come from European countries, we found that the mean concentration of Cd was similar to values reported in Greece (0.19â±â0.15 μg/L)18, lower than those in Spain (0.4â±â1.6 μg/L)19 and previously reported in Poland (2.114â±â2.112 μg/L)20, but higher than in Sweden (0.086â±â0.045 μg/L)21. We did not find any relationship between Cd concentration and maternal factors, including anthropometric data and current dietary intake, however significant positive correlation was reported for habitual salty snacks intake (râ=â502, pâ=â0.0047). The main dietary sources of Cd are plant-based food, mainly potatoes, which are the basis for chips production, which may explain our results22. Contrary to these findings, Motas et al.19 reported that Cd concentration was negatively associated with maternal age (pâ=â0.012) and current weight of the baby (pâ=â0.038) and positively correlated with maternal current or past smoking habits (pâ=â0.014) and following vegetarian diets (pâ<â0.01). In turn, Leotsinidis et al.18 reported higher Cd content in samples of mothers with high vegetable intake. On the other hand, Nakhaee et al.17 and Ursinova and Masanova23 did not observe any associations between maternal factors and Cd content in HM.
Considering the As level in HM, the literature data are rather limited. We detected As in all HM samples, contrary to American24 and Spanish19 studies, in which this metal was detectable in 56% and only 12% of analyzed samples, respectively. One of the reasons for this discrepancy are different LOD for this metal, which was 0.0012 μg/L in our study and 0.22 μg/L in the American study. Motas et al.19 did not provide the LOD. Interestingly, the mean concentration of As in our study (0.7â±â1.0 μg/L) was lower than those reported by Motas et al. (0.9â±â2.7 μg/L)19 who found a large variance in As levels in the tested population. Similarly to our results, a recent Swedish study21 showed that As was detected in all tested samples of HM (0.55â±â0.7 μg/L; LODâ=â0.007 μg/L). The main maternal dietary sources of As are drinking water, grain, and grain-based products, mainly rice-based products25. In our study, we observed that As content in HM was weakly correlated with maternal whole-grain product consumption (râ=â0.370; pâ=â0.044). No other correlations between As levels in HM and maternal factors were found.
The levels of Pb found in our study (Table 3) were consistent with those reported from Slovakia (4.7 μg/L)23, but higher than results obtained in Turkey (2.27â±â0.36 μg/L)11, Portugal (1.55â±â1.38 μg/L)26, and Greece (0.48â±â0.6 μg/L)18. We hypothesized that Pb present in HM originated mainly from maternal bone deposits. During the second trimester of pregnancy, when the fetal skeletal is developing, Pb enters to maternal blood along with calcium mobilization from the bones. Then, Pb is resorbed from maternal bone stores to the bloodstream and HM to compensate for calcium needs during breastfeeding27. Performed in our study multivariable logistic regression analysis showed that higher HM lead concentration was predicted by maternal age (95% CI [0.94â0.97]) and intake of fish (95% CI [1.01â1.03]) and vegetables (95% CI [1.02â1.06]). Considering food consumption, other authors found that intake of cereals, fish28, red meat, cheese, rice17, coffee, and dairy products29 affects Pb content in HM. In turn, Nassir et al.30 and Cherkani-Hassani et al.15 reported that the milk of mothers who consume well water had a lower concentration of Pb in comparison to those who used tap water for drinking.
Sharma et al.31 reported a significant decline in Hg concentration in HM between 1966 and 2015. These global findings are consistent with observations of several national monitoring studies, eg. in Sweden32, Slovakia23 or the Republic of Korea33, as well as a recent review concerning biomonitoring of terrestrial environments34 and extensive study covering ten years of official monitoring of food of animal origin in Poland35. Mercury level was previously reported in only one Polish study36 which revealed a significantly higher mean concentration (4.4 μg/L). However, it is worth mentioning that the standard deviation was very high (4.4â±â9.1 μg/L), which means that the values varied greatly. In Germany37, the Hg concentration ranged fromâ<â0.2 to 6.86 μg/L (median 0.37 μg/L), which is similar to our results (mean value was 0.5â±â0.1 μg/L), whereas in Austria28 the mean concentration was higher and mean values ranged from 1.07 to 2.17 μg/L (depending on the region). In Slovakian samples23 mean Hg concertation amounted to 0.94 μg/L and the only factor affecting its content was the number of maternal teeth feelings (more or less than 7 tooths), 1.08 vs 0.84 μg/L, respectively (râ=â0.26; pâ<â0.01). The Austrian28 and Moroccan38 authors reported that instead of the region, the HM Hg concentration was affected by the maternal consumption of cereals and vitamin supplementation during pregnancy, whereas smoking did not influence the Hg content in HM. In our study group, positive correlations with plant-based food intake, such as fruits and nuts/seeds were observed (râ=â0.424; pâ=â0.042 and râ=â0.378; pâ=â0.039, respectively). These results have been also confirmed in multivariable logistic regression analysisâintake of fruits (95% CI [1.02â1.06]) and nuts/seeds (95% CI [1.03â1.12]) affected HM Hg concentration. Similarly, Kalemba-Drozdz36 observed higher Hg concentration in Polish milk samples from vegan mothers in comparison to omnivorous women. Our results were interesting, since in general, fruits showed lower metal accumulation compared to vegetables39. The reason given to explain this result may be the fact that in our group, maternal fruit intake was higher than consumption of vegetables (FFQ results: 17.4/20 points vs 14.2/20 points, respectively).
In Table 4 correlations between element concentrations in HM and selected maternal factors are presented.
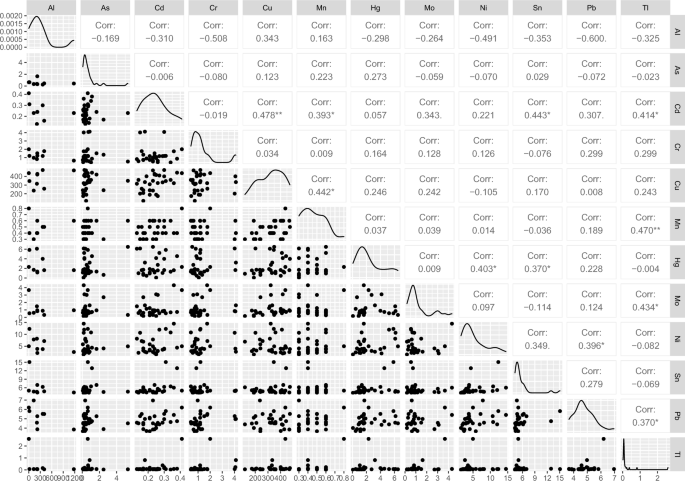
The correlation between the elements was also examined and V, Cu, Cd, B, and Pb showed normal distribution (Pearson correlation was considered). For the rest elements, considering non-normal distribution, the Spearman correlation was applied. The highest positive correlations were found between Mn and B (râ=â0.527; pâ=â0.0033); Ni and Sn (râ=â0.515; pâ=â0.004); Pb and Sn (râ=â0.513; pâ=â0.004). Pb concentration was also positively related to the content of Ni (râ=â0.493; pâ=â0.0056), Cr (râ=â0.468; pâ=â0.009), and Mn (râ=â0.411; pâ=â0.024). Interestingly, opposite to our results, in the Turkish study11 negative correlation between Pb and Cr was found (râ=ââ 0.328; pâ<â0.05). Additionally, in the present study, Cu concentration was correlated with three other elements Cd (râ=â0.478; pâ=â0.0076), Hg (râ=â0.418; pâ=â0.021), and Be (râ=â0.427; pâ=â0.037). The correlations of 11 essential and non-essential among trace elements in studied HM samples are presented in Fig. 1.
Pairways correlation coefficient of selected elements in analyzed human milk samples.
Infantsâ intake of analyzed elements
Based on the elementsâ concentration in HM and the volume of milk intake, infantsâ exposure to tested elements was calculated (Table 5). The highest mean intake was for Cu (35.24â±â12.48 μg/kg body weight/day). A similar value was for Al (22.71; 12.39â33.25 μg/kg body weight/day), however, it must be stressed that this element was detected in only 9 samples. Considering elements detected in all samples, the lowest exposure was for As (0.045; 0.025â0.08).
According to the European Food Safety Authority (EFSA)40, the information concerning Cd intake by infants is lacking. Based on the results of only two studies, infants exposure was estimated at the level of 2.61â2.74 μg/kg body weight/week (0.37â0.39 μg/kg body weight/day), which is significantly higher than the results reported in our study. It may result from the fact that in our study we included infants at the age of 4â6 weeks, whereas in the next weeks the volume of consumed milk increases which will translate into higher Cd intake. On the other hand, similar results (mean values: 0.05â0.07, depending on the maternal age) were obtained in the study performed by Shawahna et al.41, in which most of the infants were over six months of age.
European data42 suggests that Pb intake in infants ranges from 0.21 to 0.94 μg/kg body weight/day, which is similar to our results (0.351â0.833 μg/kg body weight/day). However, in the EFSA document, there is no information about the exposure of exclusively breastfed infants. In Slovakian newborns23 (milk samples were obtained on the 4th day postpartum) Pb intake was estimated at the level of 0.36â2.03 μg/kg body weight/day. Additionally, the authors reported that in two individual samples, Pb level exceeded provisionally tolerable weekly intakes (PTWI) (25 μg/kg body weight/week) established by FAO/WHO43. Nonetheless, according to EFSA Experts [EFSA, 2010] there is no evidence for a threshold for critical Pb-inducted effects and PTWI of 25 μg/kg body weight/week is no longer appropriate and should not be used. In our study, we found that infantsâ exposure to Pb was correlated with maternal frequency consumption of canned fish (râ=â0.504, pâ=â0.0045).
Based on the limited data on the occurrence of methylmercury and inorganic mercury in HM in Europe44, the exposure for a three-month exclusively breastfed infant ranged approximately from 0.09 to 0.62 μg/kg body weight/week (0.013â0.09 μg/kg body weight/day) with mean HM consumption and at mean occurrence and from 0.17 to 1.29 μg/kg body weight/week (0.024â0.18 μg/kg body weight/day), respectively. For infants with high milk consumption (about 1200 mL/day), the dietary exposure to methylmercury ranged from 0.14 to 0.94 μg/kg body weight/week (0.02â0.13 μg/kg body weight/day) and inorganic mercury â 0.25 to 1.94 μg/kg body weight/week (0.04â0.28 μg/kg body weight/day). The established tolerable weekly intake (TWI) for methylmercury and inorganic mercury is 1.3 μg/kg body weight and 4 μg/kg body weight, respectively44. In our study, we assessed the total Hg intake and it was 0.196â0.749 μg/kg body weight/week (0.028â0.107 μg/kg body weight/day) which means that the mean intake of Pb was below TWI for both forms of Hg.