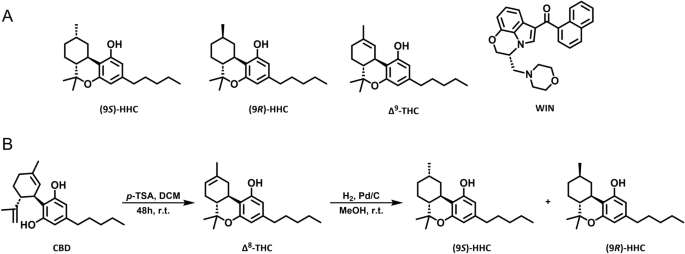
CB1R ligands
WIN 55,212-2 mesylate (WIN) was obtained from Tocris R&D (USA). Î9-tetrahydrocannabinol (Î9-THC) was synthesized as described previously23. Hexahydrocannabinol (HHC) was synthesized as described below.
Synthesis and purification of HHC
Commercially-available CBD isolate (CBDepot, Czech Republic), p-toluenesulphonic acid (P-Lab, Czech Republic), and 5% palladium on activated charcoal (Merck KGaA, Germany) were used for the reaction. Solvents were purchased from a local distributor (Lach-Ner, Czech Republic) and were used without further purification. Solvents were evaporated using a vacuum rotary evaporator. Argon (5N) was used as an inert gas, and hydrogen (3.5N) was used for the reduction. Polar silica 40â63 µm (Merck KGaA, Germany) was used for â8-THC purification. The Aldrich® Kugelrohr⢠short-path distillation apparatus (Merck KGaA, Germany) was used for HHC vacuum distillation. HHC epimers were separated using COMBIFLASH RF200 UV/VIS (Teledyne ISCO, United States) and RediSep Gold® Silica Gel Disposable Flash Columns (Teledyne ISCO, United States). HPLC/UV spectra were measured using LC/MS Agilent Technologies, 1290 Infinity DAD. The ratios of HHC epimers were determined based on signal characteristics in 1H NMR spectra (δ 3.03 ppm for (9R)-HHC and δ 2.87 for (9S)-HHC).
The scale of the reaction ranged from 10 g of CBD up to 1 kg. CBD was dissolved in DCM to achieve a concentration of 50 g/L. For every gram of CBD, 0.5 g of p-toluenesulphonic was added. The mixture was flushed with argon and stirred for 48 h at room temperature. The reaction mixture was filtered through the silica column using 3 g of silica for every gram of CBD. The silica was washed with DCM until no more product was eluted. The solution of â8-THC in DCM was concentrated to 1/10 of its original volume. An equal amount of MeOH was added diluting the solution approximately two times and the solution was evaporated once again to half of its volume. This procedure was repeated until no DCM signal (δ 5.30 ppm) was present on 1H NMR. The resulting mixture of â8-THC and MeOH was used for the reduction without further purification.
The corresponding conditions are listed in the Table 1. Palladium on activated charcoal was added to the solution of â8-THC in MeOH. The reaction vessel was flushed with argon and then the argon was replaced by hydrogen. The mixture was stirred, and the pressure of hydrogen was maintained at around 1 atm. The mixture was filtered through celite and the celite was washed with MeOH until no more product was eluted. The MeOH was evaporated and the crude HHC was vacuum distilled using Kugelrohr⢠(220 °C, 0.4 torr). A mixture of epimers (9R/S)-HHC (HPLC/UV purity 96%) was produced by this procedure. Samples of pure (9R)-HHC and (9S)-HHC were obtained from a 3:2 mixture (entry 2) using FLASH chromatography (hexane: t-BuOMe, 1â2%).
NMR characterization of HHC epimers
The NMR spectra were measured with Agilent 400 MR DDR2 (Agilent Technologies Inc., United States) using CDCl3 (Merck KGaA, Germany) as a solvent and referenced on residual CDCl3 signal (1H δ 7.26 ppm). The spectra of corresponding epimers were identical to NMR spectra published by Russo et al.11.
(9S)-HHC
1H NMR (400 MHz, CDCl3) δ 6.25 (d, Jâ=â1.6 Hz, 1H), 6.07 (d, Jâ=â1.6 Hz, 1H), 4.70 (s, 1H), 2.91â2.85 (m, 1H), 2.71â2.64 (m, 1H), 2.47â2.37 (m, 2H), 2.15â2.07 (m, 1H), 1.69â1.61 (m, 3H), 1.56 (p, Jâ=â7.6 Hz, 2H), 1.51â1.44 (m, 1H), 1.36 (s, 3H), 1.35â1.27 (m, 6H), 1.13 (d, Jâ=â7.3 Hz, 3H), 1.09 (s, 3H), 0.88 (t, Jâ=â7.0 Hz, 3H).
(9R)-HHC
1H NMR (400 MHz, CDCl3) δ 6.25 (d, Jâ=â1.7 Hz, 1H), 6.08 (d, Jâ=â1.6 Hz, 1H), 4.69 (s, 1H), 3.06â3.00 (m, 1H), 2.49â2.38 (m, 3H), 1.88â1.81 (m, 2H), 1.68â1.59 (m, 1H), 1.58â1.50 (m, 2H), 1.49â1.38 (m, 1H), 1.37 (s, 3H), 1.35â1.24 (m, 4H), 1.17â1.02 (m, 2H), 1.07 (s, 3H), 0.94 (d, Jâ=â6.6 Hz, 3H), 0.88 (t, Jâ=â7.0 Hz, 3H), 0.83â0.74 (m, 1H).
Cell culture and transfection
Human Embryonic Kidney 293 (HEK293) cells (ATCC, USA, CRL-1573) were cultured in high glucose Dulbeccoâs Modified Eagleâs Medium (DMEM) (Sigma) supplemented with 10% fetal bovine serum (Gibco) at 37 °C, 5% CO2 in the air, and 95% humidity. The cells were plated in 96-well plates (Greiner BioOne, UK) at 50,000 cells per well and transfected with 150 ng of DNA per well using Lipofectamine 2000 (Invitrogen) according to the manufacturer’s instructions. The transfected cells were tested 24 h after transfection.
Bioluminescence resonance energy transfer assay
Bioluminescence resonance energy transfer (BRET) assay was used to measure CB1R-induced G protein dissociation and β-arrestin interaction with CB1R, as described previously24,25. To evaluate G protein dissociation, we transfected the cells with Gαi1-Rluc8 or GαoA-Rluc8, Gβ2-Flag, Gγ2-EYFP, and SNAP-CB1R plasmids in a mass ratio of 1:1:1:2. To measure β-arrestin2 interaction with CB1R, we transfected the cells with β-arrestin2-Rluc and CB1R-EYFP plasmids in a mass ratio of 1:2. To study GRK3-CB1R interaction, the cells were transiently transfected with GRK3-Rluc8 and CB1R-EYFP plasmids (1:2 ratio). Before the measurements, the transfected cells were washed with phosphate-buffered saline (137 mM NaCl, 2.7 mM KCl, 8 mM Na2HPO4, 1.8 mM KH2PO4) and incubated in Tyrodeâs solution (137 mM NaCl, 0.9 mM KCl, 1 mM MgCl2, 1 mM CaCl2, 11.9 mM NaHCO3, 3.6 mM NaH2PO4, 5.5 mM D-glucose, 25 mM HEPES, pH 7.4) at 37 °C for at least 30 min. Next, we added coelenterazine h (NanoLight) at a final concentration of 5 µM to the cells, followed by the addition of increasing concentrations of compounds (9S)-HHC, (9R)-HHC, Î9-THC, WIN, or their vehicles. BRET donor and acceptor emission was measured 12 min after the addition of the compounds using Mithras LB940 plate reader (Berthold Biotechnologies, Germany). The BRET ratio was obtained by dividing the acceptor emission (540â±â20 nm) by the donor emission (480â±â10 nm). After subtracting the BRET ratio of the vehicle addition from the BRET ratio of the compounds, we obtained deltaBRET (ÎBRET). Data analysis was performed using GraphPad Prism 9.3.1 for Windows (GraphPad Software, USA). The concentrationâresponse curves were fitted using a non-linear regression function.
CB1R internalization assay
Cell surface receptor internalization was studied using the Homogenous Time-Resolved FRET (HTRF) technology as described previously26. First, HEK293 cells were seeded on a 96-well plate (Merck, Germany) and transiently transfected with SNAP-tagged CB1R plasmid together with empty vector pRK6 (1:2 DNA mass ratio) using Lipofectamine⢠2000 (Thermo Fisher Scientific) according to the manufacturer’s protocol. Twenty-four hours post-transfection, the cell culture medium was removed, and the cells were labeled with 100 nM SNAPLumi4-Tb (PerkinElmerâCisBio, France), diluted in Tag-lite labeling medium (PerkinElmerâCisBio, France) and incubated for 1 h at 37 °C, 5% CO2. Subsequently, labeled cells were washed four times with Tag-Lite buffer solution. The receptor internalization experiment was performed by adding Tag-lite buffer containing 24 μM fluorescein (Merck, Germany) and corresponding CB1R agonist or vehicle (dimethyl sulfoxide, or in the case of Î9-THC, ethanol). HTRF signal was recorded over 60 min at 37 °C using the Mithras LB 940 microplate reader (Berthold Technologies, Germany) equipped with the HTRF module and relevant filters. The donor fluorophore (terbium cryptate) was excited at 340â±â26 nm and emission was measured at 520â±â10 nm. The acceptor (fluorescein) emission was measured at 620â±â10 nm. The HTRF ratio was calculated as the donor emission divided by the acceptor emission multiplied by 10,000.
Extracellular signal-regulated kinases 1/2 phosphorylation assay
Phosphorylation levels of endogenous extracellular signal-regulated kinases 1/2 (ERK1/2) were detected using the Phospho-ERK1/2 (Thr202/Tyr204) kit (Cisbio Bioassays, France). The transfected cells plated in 96-well plates (Greiner BioOne, UK) were serum-starved for 16 h prior to the experiment in serum-free DMEM media. Afterwards, the cells were stimulated for the indicated times by CB1R ligand diluted in serum-free DMEM and then lysed in 50 μl of supplemented lysis buffer. After homogenization, 16 μl of the cell lysate was transferred from the 96-well plate to a 384-well black plate (Greiner BioOne, UK) and incubated with 4 μl of detection buffer containing anti-ERK1/2-Eu3+cryptate and anti-Phospho-ERK1/2-d2 for at least 4 h in dark. The fluorescence emissions at 665 nm and 620 nm were read on HTRF® compatible Mithras LB 940 microplate reader (Berthold Technologies, Germany). Data are presented as the ratio of 665 nm emission and 620 nm emission multiplied by 10,000.