Gliomas are a family of tumors whose cells of origin may be neural stem cells (NSCs), NSC-derived astrocytes, and oligodendrocyte precursor cells (OPCs). They account for about 80% of all malignant brain tumors in the central nervous system (CNS) [1,2,3]. Treatment and prognosis of gliomas depend on the glioma type and on several prognostic factors [4]. However, despite recent advances, treatment of gliomas is often problematic and ineffective. The survival of high-grade glioma patients is less than 15 months even after surgery, and the overall 5-year survival rate is below 7% [2, 5]. The current standard of care for high-grade gliomas consists of maximal safe resection of the tumor followed by chemoradiation therapy using the alkylating agent temozolomide [6]. Several bottlenecks affect the treatment of glioma. The removal of the tumor is hampered by the infiltrative nature of most gliomas, and by the difficult and unreliable identification of the tumor margins [7]; radiotherapy has efficacy concerns, in addition to acute, early-delayed and late-delayed neurological side effect induced in CNS [8]. Bevacizumab (Avastin) and temozolomide are the most used drugs against high-grade gliomas. They show a good safety profile and demonstrated some clinical benefit, although only little prolonged progression-free survival or overall survival improvement could be demonstrated [6, 9, 10]. Further, high-grade gliomas inevitably recur, because they are often inherently resistant to therapy, their genetic heterogeneity hampers targeting of single oncogenic pathway, and they are in a microenvironment that includes microglia that can be tumor-supportive and that influences responses to therapy [11,12,13,14,15].
In addition, the blood-brain barrier (BBB) is an obstacle that almost all chemotherapeutic drugs need to overcome. The BBB is a complex, extensive, multicellular protective cell barrier between the CNS and the peripheral blood circulation. It consists of brain capillary endothelial cells, a basement membrane, pericytes, astrocytes, and tight junctions. The BBB maintains CNS homeostasis by tightly controlling the passage of specific nutrients, such as amino acids, glucose, nucleosides, and fatty acids, and restricting the passage of harmful xenobiotic molecules, such as neurotoxic agents, from the vasculature into the extracellular fluid of the CNS. The BBB prevents almost all macromolecules and at least 98% of small-molecule drugs, including most chemotherapeutics, from entering the brain. Therefore, drug delivery strategies aimed at significantly improving BBB crossing and drug accumulation in gliomas are highly valuable against brain tumors and diseases [16,17,18].
Nanocarriers show promising clinical potential for glioma targeting. Most nanocarriers studied for brain delivery are surface-modified to exploit cellular receptor-, transporter-, or adsorption-mediated mechanisms for drug delivery. The receptor-mediated transcytosis strategy is the most promising one, as it employs highly expressed endocytosis-related receptors in BBB endothelial cells, such as transferrin receptors (TfRs), insulin receptor, lactoferrin receptor, low-density lipoprotein receptors, folate receptor, melanotransferrin, CD98, and a variety of antibodies and ligands [16, 18,19,20,21,22,23]. Nanocarriers decorated with appropriate ligands could thus bind these receptors and cross the BBB via receptor-mediated transcytosis.
TfRs are the most studied and validated target protein for brain delivery approaches, and many technologies targeting TfRs have been used, mostly using antibodies [18]. The ferritin/transferrin receptor TfR1 (CD71) is expressed in normal cells such as erythrocytes, hepatocytes, and intestinal cells, where it is needed for iron metabolism, since it binds specifically and with high affinity transferrin (Tf) and the H subunit of ferritin and allows internalization of these iron-binding proteins [24,25,26,27,28]. In energy-requiring cells, such as brain endothelial cells, and in rapidly proliferating cells such as cancer cells, the expression level of TfR1 is significantly elevated; the expression of TfR1 on the surface of many types of cancer cells is up to 100-fold higher than that of normal cells [29, 30]. The high expression of TfR1 in both glioma cells and brain endothelial cells makes TfR1 an ideal target receptor for drug delivery across the BBB and to the glioma. Due to the high concentration of endogenous Tf in the bloodstream, competition may occur with Tf-modified nanocarriers, that often exhibit unsatisfying targeting ability and safety [23].
Ferritin (Ft) is a spherical nanocage composed of 24 subunits (heavy (H) or light (L)), able to reversibly assemble and disassemble in a pH-dependent fashion, with a hollow center for storing up to 4500 iron (Fe3+) ions [31, 32]. Ft-based nanocarriers have been developed, mostly using their cavity to incorporate drugs and other payloads, in order to deliver them to tumors. Ferritin can be produced with high purity and is inexpensive, non-immunogenic, biodegradable, highly soluble and able to protect the payload from reducing agents and from metabolism [33], and is able to pass the BBB via TfR1-dependent transcytosis [24, 34]. However, the delivery of drugs into the CNS using Ft-based nanocarriers is less investigated, with only a few studies using doxorubicin- and paclitaxel-loaded ferritin for glioma treatment [24, 35,36,37]. All of these studies were performed using the H chain of Ft (HFt), the only one specifically able to bind to TfR1. Notably, HFt binds to a different epitope than Tf, so its binding (and therefore, its ability to cross the BBB and enter glioma cells) cannot be competitively inhibited by endogenous Tf [26, 28].
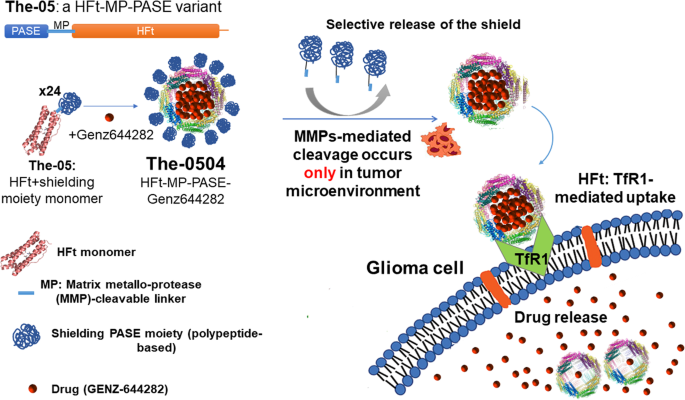
We therefore designed a stimuli-sensitive HFt-based nanocarrier, in which HFt is joined with a peptide sequence specifically cleavable by metalloproteases 2 and 9 (MP) to a shielding polypeptide named PASE, which is composed of only proline (P), alanine (A), serine (S), and glutamic acid (E) (Fig. 1). The PASE polypeptide increases HFt water solubility, causes a tenfold decrease in TfR binding affinity, and extends the half-life of the nanocarrier in the bloodstream compared to native HFt [38]. At the tumor site, the PASE-linked MP sequence is selectively cleaved off by matrix metalloproteases 2 and 9 of the tumor microenvironment, leading to HFt unmasking, and hence to binding to TfR1, selective internalization in the cancer cells, and tumor killing. The HFt-MP-PASE protein (also called The-05) incorporating doxorubicin, mitoxantrone and the potent non-camptothecin topoisomerase I inhibitor Genz-644282 was proved to be efficacious against several tumor models of different origin and well-tolerated in multiple-cycle repeat-dose study in both mice and rats [25, 38,39,40].
The-05 is composed by polypeptide comprises the N-terminal shielding PASE moiety (blue), a matrix metalloprotease (MMP)-cleavable linker (clear blue) and a C-terminal human ferritin heavy chain (HFt, orange), that fold into a The-05 monomer. In the presence of the drug Genz-644282, using a simple pH-dependent association-dissociation protocol, the monomer assembles into a The-0504 24-mer, where the drug is encapsulated into the stimuli-sensitive protein, that can be administered to the tumor mice model. In the tumor microenvironment MMP-mediated cleavage of the PASE shield occurs, the The-0504 is internalized by glioma cells via TfR1, and the drug is released into glioma cells, where it inhibits topoisomerase I.
The compound formed by The-05 and Genz-644282 is named The-0504, and here we evaluated its activity on murine glioma cells, both in vitro and in in vivo model. In order to be able to use our stimuli-sensitive delivery system, first we measured the levels of TfR1 in these glioma cells compared to normal brain cells. In addition, the affinity between HFt and both human and mouse TfR1 were evaluated by Surface Plasmon Resonance experiments.
Then, we tested the ability of our nanovector system to reduce glioma growth and increase overall survival. To this end, we evaluated two different administration routes: intravenous and intranasal. The nose-to-brain route has several advantages, since it is a highly accessible, non-invasive pathway of delivery, characterized by rapid onset of action and high cerebral bioavailability, that has been tested in many investigational studies in the past five years and has the potential to pave the way for a brighter future in the management of brain diseases; a few approved nasal formulations exist for brain disorders such as opioid overdose, migraine, depression, pain and anxiety [16]. The nose-to-brain route is easily accessible, highly vascularized, and physically short. It is directly connected to the brain via the olfactory pathway, the respiratory pathway, the systemic pathway, and the nasopharynx-associated lymphoid tissue. Importantly, it partially bypasses the BBB [16, 41]. However, intranasal administration has limitations, as the limited administration volume, mucus layer, and the possibility that drugs can be degraded and/or fail to reach the site of action. This has led to many studies on the implementation of nanoparticle systems, based on many different carriers such as liposomes, micelles and polymeric nanoparticles, to overcome these limitations and accurately deliver drugs to the site of action [16]. For these reasons, we decided to preliminarily investigate the possible use of The-0504 in brain tumors via the nose-to-brain route using an orthotopic model of murine glioma.