Strains and plasmids
Escherichia coli strain TOP10 was purchased from Invitrogen. pBad43 plasmid was kindly provided by Michael Deghelt from Institut de Duve, UCLouvain, Brussels.
Cloning of pbp-a gene in the rhamnose operon of the chromosome
The detailed protocol of genome engineering for building scarless gene libraries in RhaA locus of E. coli chromosome using CRISPR-Cas9 technology and λ-Red recombineering system is described in34. Briefly, recombinant pbp-a gene fused to DNA encoding the signal peptide of DsbA and a 6 × His tag fused at the amino- and carboxy-terminus, respectively, was replaced with the rhaA gene at the second position of the polycistronic operon of rhamnose in the genome of E. coli.
Cloning of pbp-a genes into low- and high-copy plasmids
The gene encoding pbp-a was amplified and assembled into pBAD43 plasmid thanks to the overlapping sequences integrated in primers followed by Gibson assembly. Following primer pairs were used for PCR amplification of pbp-a gene variants and pBAD43 and pBAD plasmid backbone. Overlapping sequences, upstream and downstream of pbp-a gene, are indicated as underlined and bold, respectively:
Pair 1: pbp-a fw: ttttagcgtttagcgcatcggcggc.
pbp-a rv: ttggctttcgcccattcaactcagtgatgatgatgatgg.
Pair 2: pBAD-BackBone-PBPs fw: cactgagttgaatgggcgaaagccaatctagagtcgacctgcaggc.
pBAD-BackBone-PBPs rv: cgatgcgctaaacgctaaaactaaaccagccagcgccag.
The purified PCR products were inserted into pBAD plasmid backbones by mixing 15 μL of Gibson Master Mix, together with 50–100 ng of the linear vector backbone with a 2–3 folds molar excess of the insert, 0.5 μL of DpnI (to digest the remaining original (methylated) plasmid template), and ddH2O up to 20 μL. The assembly mix is then incubated for 1 h at 50 °C and the assembled fragments (circularized plasmid) can be detected on agarose gel. 1 μL of the assembly mix is electroporated into 50 μL of E. coli TOP10 electrocompetent cells (Invitrogen). Transformants are incubated at 37 °C, for 1 h before plating all the cells on LB plates containing spectinomycin and glucose (for repression of PBP-A expression) followed by overnight incubation 37 °C. Insertion of pbp-a genes in pBAD43 plasmid was verified by colony PCR and confirmed by sequencing of the PCR products from random clones. To avoid revertant selection and adaptation against toxicity of PBP-A, analyses were confirmed with fresh transformants and minimum freezing–thawing cycles.
Catalytic S61A mutant of PBP-A was prepared by site directed mutagenesis via QuickLib protocol35 using the following primers. The substitution and, overlapping sequences between primers are indicated as underlined and bold, respectively:
PBP-S61A_fw: AATGTCGGCGGTGACCAAGTGTTTCCGGCGGCCGCTACCATTAAATTCCCGATCCTGG.
PBP-S61A_rv: CCGGAAACACTTGGTCAC.
Lysine at positions 104 and 212 were mutated to Glutamic acid by incorporating mutations on primers followed by two PCR reactions on pBAD43_PBP-As template. Reaction 1 to amplify pbp-A gene from position 104 to 212 and reaction 2, for amplification of the remaining backbone of pBAD43_PBP-As. The PCR products of reaction 1 and 2 were purified and then assembled (thanks to overlap sequences incorporated into primers), by mixing 3–4 folds molar excess of the shorter fragment (reaction 1) with 50–100 ng of longer backbone (reaction 2) and 0.5 μL of DpnI (20 U/μL) into 15 μL Gibson master mix (100 mM Tris–HCl pH 7.5, 10 mM MgCl2, 0.2 mM of each four dNTPs, 1 mM NAD+, 15% (w/v) PEG-8000, T5 exonuclease (2 U/mL), Phusion DNA polymerase (33 U/mL), and Taq DNA ligase (1666 U/mL)), followed by transformation into E. coli competent cells.
Following primer pairs were used for the two reactions. Overlapping sequences, at regions 104 and 212 are indicated as underlined and bold, respectively:
Reaction 1
Pbb-a_K104E fw:
ATTGCCCCGGAAGCAGGCACCCTGCAGTATCAAGAACCGAATTCACAATACGCAG
Pbb-a_K212E rv: CGGCAGCAGGGTATTGGTAACGGTG.
Reaction 2
Pbb-a_K212E fw:
ACCGTTACCAATACCCTGCTGCCGGCCGGTCTGGGTGAAGGTGCAACGATCGCTCATAAAACC
Pbb-a_K104E rv: ATACTGCAGGGTGCCTGCTTC.
Preparation of cell lysates
Periplasmic extraction: After induction of PBP-A expression for 3–4 h, E. coli cultures (10 mL) were harvested at 4400 × g for 10 min, supernatants were discarded, cells were resuspended in 0.33 mL of 20% (w/v) sucrose in 20 mM Tris–HCl, pH 8.0, and 33 µl of 0.1 M EDTA, pH 8.0 was added. Samples were incubated at room temperature for 10 min followed by 10 min centrifugation of cells at 6000×g. Supernatants were discarded, and the pellets subjected to osmotic shock by resuspending in 0.5 mL of 5 mM MgSO4. The mix was incubated at 4 °C for 10 min followed by 10 min centrifugation at 10,000×g. The supernatants were recovered as periplasmic extracts. The pellets could be used to recover the cytoplasmic content.
Complete cell lysis: Pellet of 10 ml bacterial culture was resuspended in 500 μL of lysis buffer containing 10% glycerol, 0.1% Triton X-100, 100 µg/mL lysozyme, 1 mM EDTA and 3 U DNAse. The cell suspension was incubated at room temperature for 30 min and sonicated 5 times for 20 s until the samples were no longer viscous and a clear lysate was obtained. After centrifugation at 4 °C and 12,000×g for 10 min, the supernatant was collected as the soluble cell lysate.
Insoluble fraction: The pellet remaining from the cell lysis preparation was considered as insoluble fraction and it was suspended in 500 μL of PBS, pH 7.0 for further analysis.
Western blot analysis
Volumes of culture were normalized to the same OD600, by diluting the denser cultures, to subject similar numbers of cells to the periplasmic extraction. Protein samples are run on 4–20% iD PAGE Gel (Eurogentec) and transferred onto a 0.2 μm nitrocellulose membrane using the Trans-Blot® Turbo™ Transfer System at constant 2.5 A and up to 25 V for 5 min. Then the membrane is incubated in blocking solution (5% non-fat dry milk, 0.2% Tween, PBS buffer pH 7.2) for 1 h at room temperature under gentle agitation to prevent non-specific antibody binding. The membrane is incubated for 1 h in primary antibody (5 mg/mL of rabbit anti-His (Sigma) diluted 1:2000 in blocking solution). The membrane is washed 3 times with the blocking solution for 10 min under agitation to remove unbound primary antibody. The tray is changed to decrease the background effect of primary antibodies and the membrane is incubated for 1 h in secondary antibody (Peroxidase AffiniPure Donkey Anti-rabbit IgG (H + L)), Jackson ImmunoResearch, UK). Before proceeding to detection, the membrane is washed 2 times with the blocking solution for 10 min under agitation followed by one time washing with PBS buffer. For chemiluminescence detection of signals, the membrane is soaked in substrates mixture of substrate A and substrate B (Pierce™ ECL Plus Western Blotting Substrate, Thermofisher) with a ratio of 40:1 as suggested by supplier. The signals are detected on Amersham™ Imager 600 (GE Healthcare).
Purification of PBP-A proteins
PBP-A proteins were purified from total soluble lysate by affinity chromatography thanks to the 6-histidine (6 × His) tag fused to the C-terminal. Briefly, the total soluble lysate of E. coli cells expressing different PBP-As were loaded onto a Ni-Sepharose column. After several washes by washing buffer (20 mM HEPES, 20 mM Imidazole, 150 mM NaCl, pH 8.0) 4–5 times the column volume, a stepwise gradient of imidazole (50–500 mM in 50 mM steps) was applied to elute the variants.
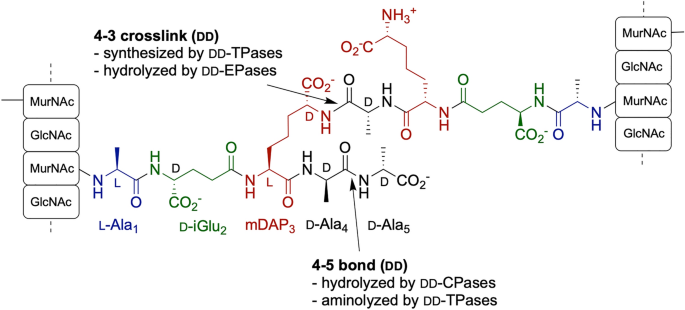
Synthesis of mSDAP-containing peptides
Pentapeptide substrates were synthesized by solid-phase peptide synthesis (SPPS) using standard fluorenylmethoxycarbonyl (Fmoc) chemistry on a Symphony synthesizer (Protein Technologies Inc): Ac-l-Ala-d-iGlu-l-Cys-d-Ala-d-Ala-OH and Ac-l-Ala-d-iGln-l-Cys-d-Ala-d-Ala-OH. Pre-loaded Fmoc-d-Ala-Wang resin (Novabiochem) was used as the support. To modify l-cystein into mSDAP aminoacid, peptides were mixed with a 25-fold excess of H-β-Chloro-d-Ala-OH (Bachem) in water (pH 8 with N-Methylmorpholine) and reacted for 4 h at 45 °C. The peptides obtained were purified by reverse-phase HPLC to > 98% purity and characterized by mass spectrometry.
PBP-A activity assay using synthetic peptides as substrates
A reaction containing 5 μL of enzymes (0.15–0.2 mg/mL), 10 μL of 5 mM synthetic pentapeptides (Acetyl-l-Ala-d-iGlu-mSDAP-d-Ala-d-Ala or Acetyl-l-Ala-d-iGln-mSDAP-d-Ala-d-Ala) and 10 μL HEPES buffer (20 mM, pH 7.4), incubated for 1 h at room temperature, followed by analysis by nanoUPLC-MS. For inhibition of PBP-A, 10 μL of 100 mM penicillin G (PenG), prepared in 20 mM HEPES pH 7.4, was used instead of the buffer in the above-mentioned reaction.
Analysis of peptides by nanoUPLC-MS
In-solution samples are prepared by adding 1% trifluoroacetic acid (TFA) to the samples to reach pH below 7.0.
Peptide separation using nanoUPLC
Peptide mixture was separated by reverse phase chromatography on a NanoACQUITY UPLC MClass system (Waters) working with MassLynx V4.1 (Waters) software. 5 μL of each were injected on a trap C18, 100 Å 5 μm, 180 μm × 20 mm column (Waters) and desalted using isocratic conditions with at a flow rate of 15 μL/min using a 99% formic acid and 1% (v/v) ACN buffer for 3 min. Peptide mixture was subjected to reverse phase chromatography on a C18, 100 Å 1.8 μm, 75 μm × 150 mm column (Waters) PepMap for 35 min at 35 °C at a flow rate of 300 nL/min using a two parts linear gradient from 1% (v/v) ACN, 0.1% formic acid to 40% (v/v)) ACN, 0.1% formic acid for 15 min and from 40% (v/v) ACN, 0.1% formic acid to 85% (v/v)) ACN, 0.1% formic acid for 10 min. The column was re-equilibrated at initial conditions after washing 30 min at 85% (v/v)) ACN, 0.1% formic acid at a flow rate of 300 nL/min. For online LC–MS analysis, the nanoUPLC was coupled to the mass spectrometer through a nano-electrospray ionization (nanoESI) source emitter.
LC-QTOF-MS/MS analysis (DDA)
DDA (Data Dependent Analysis) analysis were performed on an SYNAPT G2-Si high definition mass spectrometer (Waters) equipped with a NanoLockSpray dual electrospray ion source (Waters). Precut fused silica PicoTipR Emitters for nanoelectrospray, outer diameters: 360 μm; inner diameter: 20 μm; 10 μm tip; 2.5″ length (Waters) were used for samples and Precut fused silica TicoTipR Emitters for nanoelectrospray, outer diameters: 360 μm; inner diameter: 20 μm; 2.5″ length (Waters) were used for the lock mass.solution. The eluent was sprayed at a spray voltage of 2.8 kV with a sampling cone voltage of 25 V and a source offset of 30 V. The source temperature was set to 80 °C. The cone gas flow was 20 L/h with a nano flow gas pressure of 0.4 bar and the purge gas was turned off. The SYNAPT G2Si instrument was operated in DDA (data-dependent mode), automatically switching between MS and MS2. Full scan MS and MS2 spectra (m/z 50-2000) were acquired directly after injection to 35 min in resolution mode (20,000 resolution FWHM at m/z 400) with a scan time of 0.1 s. Tandem mass spectra of up to 10 precursors were generated in the trapping region of the ion mobility cell by using a collision energy ramp from 17/19 V (low mass, start/end) to up to 65/75 V (high mass,start/end). Charged ions (+ 1, + 2, + 3, + 4) are selected to be submitted to the MS/MS fragmentation over the m/z range from 50 to 2000 with a scan time of 0.25 s. For the post-acquisition lock mass correction of the data in the MS method, the doubly charged monoisotopic ion of [Glu1]-fibrinopeptide B was used at 100 fmol/μL using the reference sprayer of the nanoESI source with a frequency of 30 s at 0.5 μL/min into the mass spectrometer.
ESI-QTOF data processing
Data were processed with MassLynx V4.1(Waters). Spectrum for each sample were extracted from the TIC (Total Ion Chromatogram) and submitted to a search of masses of interest.
Activity assay on purified PG or muropeptides of E. coli
HPLC based activity assays were carried out in a final volume of 50 µL and in buffer containing 20 mM buffer agent (HEPES or NaAcetate), 100 mM NaCl, 0.05% (w/v) Triton-X 100 (reduced), 10 µL of the substrate and 5 µM of protein(s). HEPES/NaOH was used for reactions at pH of 7.5, NaAcetate was used for reactions at pH 5.0. The reaction mixture was incubated for 2 h in a thermoshaker at 37 °C, 900 rpm for the indicated time. The reaction was stopped by boiling the samples for 10 min at 100 °C. For standard activity assays on PG, purified PG sacculi was added to the reaction mixture and after the incubation the cellosyl digestion followed overnight. Activity assays on muropeptides were performed on predigested PG sacculi with cellosyl. The pH value of the muropeptides was adjusted before they were added to the reaction mixture. The reaction products were reduced with sodium borohydride and analyzed by reversed phase HPLC (see below).
Assays of PBP-As with PG of E. coli
PG from the E. coli TOP10 and CS703-1 strains were isolated as previously published36. A mixture containing 20 mM NaAcetate pH 5.0, 100 mM NaCl, 0.05% (w/v) Triton X-100 and 5 µM of each PBP-A protein was incubated for 2 or 6 h at 37 °C. After boiling, the samples were digested overnight with cellosyl, reduced with sodium borohydride, and the muropeptides were analyzed by reversed phase HPLC (see below).
Preparation of PG and muropeptides (cellosyl products)
PG sacculi were isolated from E. coli cells as described36. Purified PG from E. coli CS703-1 and BW25113∆6LDT were mixed with cellosyl (0.5 µg/mL) in 80 mM sodium phosphate, pH 4.8 and incubated overnight in a thermal shaker at 37 °C and 900 rpm. The reaction was stopped by boiling and the sample was centrifuged for 10 min at 10,000×g (ambient temperature) to separate the soluble muropeptides (supernatant) from insoluble material. The muropeptides were either used as substrate in enzymatic activity assays or analyzed by reversed phase HPLC.
Reduction of muropeptides with sodium borohydride
Prior to HPLC analysis, muropeptides were reduced with sodium borohydride36. Muropeptides were mixed with the same volume of 0.5 M sodium borate pH 9.0 in a 2 mL Eppendorf tube with a punctured lid. The reduction process was started by adding ~ 1 mg of sodium borohydride powder with a spatula. The samples were centrifuged for 20 min at 2000×g at ambient temperature during the reduction. The pH value of the samples was adjusted to 4–5 with phosphoric acid, the sample was centrifuged (5 min, 12,000×g, ambient temperature) and the supernatant was analyzed by HPLC.
Reversed-phase HPLC analysis of muropeptides
Reduced muropeptides were separated on a Prontosil 120-3-C18-AQ 3 µm reversed-phase HPLC column (Bischoff) as described36. The column was pre-equilibrated with solvent A (50 mM sodium phosphate, pH 4.31, 0.0001% NaN3). Muropeptides were separated at 55 °C with a 90 min linear gradient to solvent B (75 mM sodium phosphate, pH 4.95, 15% (or 30% methanol to facilitate elution of all anhydromuropeptides)). Muropeptides were detected by UV absorbance at 205 nm and analyzed by the Laura V4.2.11.129 software (LabLogic System Ltd.). Fractions were collected during the HPLC run and analyzed by tandem mass spectrometry (MS/MS) as described previously37.
Bacterial growth rate measurements
For all the bacterial cultures in this study, pre-cultures were started by inoculation of cells from a single colony into 5 ml of media containing appropriate antibiotics and 0.4% (w/v) d-glucose (for repression of leaky recombinant protein expression) and incubated overnight at 37 °C under agitation of 180 rpm.
To start the growth measurement experiment, 1 ml of pre-culture was inoculated into 100 ml of LB media without any antibiotic in a sterile 500 ml erlenmeyer flask and incubated at 37 °C under agitation of 180 rpm. When cultures reached early exponential phase (OD600 ~ 0.2), cultures were divided into half by transferring each 50 ml to a sterile 250 ml erlenmeyer. One of the cultures of each strain was induced for expression of PBP-As by adding l-arabinose (0.5%, w/v) followed by incubation for 4–5 h. OD600 of the cultures were measured over time from the start of the cultures.
To estimate fitness effect associated with expression of PBP-As in E. coli TOP10, we measured growth rate after induction of PBP-As expression and maximum optical density at 600 nm (max OD600) from growth curves of induced and basal expressions. Growth rate of each culture was estimated by measuring the slope of the growth curve in exponential phase and max OD of the cultures were simply determined as highest value(s) of absorbance at 600 nm during the growth. Fitness cost of each strain are quantified by dividing average values of growth rate and ODmax of induced cultures to the basal (non-induced) cultures as shown in following formula:
$$Ratio\, of_{{{\text{ growth rate}},{\text{ OD max}}}} = \frac{{ induced\, cultures_{{{\text{growth rate}},{\text{ OD max}}}} }}{{basal\, cultures_{{{\text{growth rate}},{\text{ OD max}}}} }}$$
The growth rate, can be estimated by the slope of the tangent line drawn to the inflexion of the growth curve at exponential phase, which can be estimated by following equation in Microsoft excel:
$$b = \frac{{\sum \left( {x – \overline{x}} \right)\left( {y – \overline{y}} \right)}}{{\sum \left( {x – \overline{x}} \right)^{2} }}$$
where b is the slope of the regression line and y values are absorbance at 600 nm (OD600) measured at time points x.
Osmotic stress assays
Hyperosmotic stress: LB growth medium was replaced with LB supplemented with high concentration of NaCl (0.75 M) and the growth was monitored over time. Growth rate and max OD600 were estimated as explained in previous section.
Hypoosmotic shock: LB growth medium was diluted by addition of sterile distilled water to growth media (1:1 dilution) at mid-exponential growth (OD600 ~ 0.4–0.5) followed by monitoring growth over time and calculation of growth rates and max OD600.
Vancomycin susceptibility spotting assay
Cultures of different bacterial strains were induced for expression of PBP-As by adding 0.5% (w/v) l-arabinose at OD600 ~ 0.2–0.3 and grown to late exponential phase (OD600 ~ 0.6–0.7). The culture volumes were normalized to the same OD600 by diluting higher density cultures with LB media. From the OD600 normalized samples, cultures were diluted by series of sequential tenfold dilutions in sterile LB media up to 10−7 and then 4 μL of each dilution were spotted on LB agar supplemented with 0, 100, 200 and 300 mg/L of vancomycin and 0.4% (w/v) l-arabinose. Plates were incubated 16–20 h at 37 C.
Growth of E. coli cultures under different pH
From pre-cultures, 250 mL cultures were started in LB media, grown until OD ~ 0.2, induced with 0.5% (w/v) l-arabinose, divided into 50 mL in different Erlenmeyer flasks, and immediately buffered by adding mixture of KH2PO4 and K2HPO4 of concentrated 1 M solutions in order to achieve LB medium with 50 mM total phosphate buffer at different pH (pH 5.8 to pH 8.0). The cultures were incubated at 37 °C under 180 rpm gentle agitation in and OD600 was measured every 20 min. The maximum OD600 was measured from as the highest value of absorbance reached during the growth in 18 h of growth.
Isolation of E. coli cells lysate for peptidoglycan analysis
From overnight pre-cultures, 4 mL was inoculated into 400 mL of LB media without any antibiotic in a sterile 2 L Erlenmeyer flask and incubated at 37 °C under agitation of 180 rpm. At early exponential phase (OD600 ~ 0.2), cultures were induced for expression of PBP-As by adding 0.5% (w/v) l-arabinose followed by incubation to the late exponential state (OD600 ~ 0.7). The cultures were rapidly cooled in an ice/water bath to 4C and cells were harvested by centrifugation at 4C, 5000 rpm. The supernatants were discarded, and each pellet was resuspended in 6 mL of ice-cold water. Each cell suspension was slowly (dropwise) added into 6 mL of pre-boiling 8% SDS solution under gentle stirring on a magnetic stirrer in an Erlenmeyer flask. Note that drop-wise addition of cell suspension into boiling SDS is important to ensure rapid disintegration of the cells that is required for the fast inactivation of the endogenous autolysins. Samples were boiled under stirring for 30 min. A few drops of water were added if excess foam developed or if the volume of the boiling solution decreased too much. The suspension should boil at low heat all the time. Finally, samples were cooled down to room temperature and transferred to Falcon tubes and kept at room temperature until further treatment and HPLC–MS analysis.
Docking and simulation details
For molecular dynamics (MD), we chose to simulate the structure of PBP-A covalently acylated with d-iGln-mDAP-d-Ala tripeptide as a minimal substrate for exploring the binding mode of d-iGln in the active site. We did not extend further the substrate (which is naturally polymeric) for minimizing calculation time and chose to run simulations with the acyl-enzyme intermediate because it is introducing a covalent constraint between the enzyme and the substrate, imposing the proximity between the last d-Ala residue and the catalytic Ser61. This constraint is minimizing conformational freedom of the peptide, therefore maximizing prediction confidence. Moreover, following the Hammond postulate, an intermediate is generally considered as more similar to the transition state than the enzyme substrate complex, so it is more relevant from a catalytic perspective.
Protein, ligand, and complex (without bond between Oγ of Ser61 and carbon atom of d-Ala of the ligands) was used in the docking procedure. The structure of PBP-A in complex with a penicillin G compound (PDB ID: 2J8Y)17 was used as the protein model. Tripeptide models were built as described in the following paragraph. Docking was performed for each system and 10.000 poses were obtained by using Rosetta 3.1338 and the following protocol39 using a Monte Carlo (MC) based multi-scale docking algorithm was employed. Backbone flexibility of protein active site was provided using backrub algorithm and hard_rep score.
500 poses with the lowest interface energy were initially selected and near attack conformation analysis (NAC) was performed with the distance criteria: ≤ 3.7 Å and angle: 100° ≥ and ≤ 130°. The poses which met the criteria were listed (nucleophilic attack criteria was also considered). As a result, the following number of poses were obtained: amidated tripeptide—117 poses, carboxylated tripeptide—6 poses, and carboxylic tripeptide—39 poses. Two models with lowest interface energy and two models with the lowest distance between Oγ atom of Ser61 and the carbon atom of d-Ala for each system were selected for further studies (Supplementary Table S7).
Initial reference structure was retrieved from the Protein Data Bank (PDB ID: 2J8YX-RAY)17 and unnecessary ligands were removed from the initial structures. The reference structure used in this study were protonated to pH of 7.4 using H++ server40. The protonation states of crucial residues were evaluated across a wider pH range (4.0–7.4) to determine their pH range for protonation and deprotonation. Tripeptide models (amidated, carboxylated, and carboxylic) were built from the reference structure using tLeaP package from Amber22 program package41. 4 fs time step was used for production runs using hydrogen mass repartitioning (HMR) implemented in ParmED (Supplementary Table S8)41,42. The Roe protocol was used which includes five-step minimization and a four-step equilibration protocol43. 1 µs long MD simulation with 300 K and NPT ensemble utilizing Monte Carlo barostat44 and Langevin temperature coupling45 with a gamma of 1.0 ps−1 was performed for each system. All MD simulations were performed utilizing the Amber22 program package41. AMBER ff19SB force field46 and TIP3P47 water model were used, and the protein structure was immersed in a truncated octahedral box with a distance at least 16 Å41.
In force field parameterization, the terminal sides of d-iGln, d-iGluH, d-iGlu, mDAP and SAP were capped with ACE and NME in order to provide charge stabilization and mimic the neighboring residues. In the substrate, acetylated d-iGln (capped with ACE) was used since there is no N-terminal there in the natural polymeric substrate. d-Ala and Ser61 were parameterized together and was named SAC. (Supplementary Table S9). The optimization calculations were performed at the HF/6-31G(d) level of theory using Gaussian 16 program package (Rev C.01)48. The restrained electrostatic potential (RESP) charges were obtained using the Merz–Singh–Kollman (MK) scheme (Iop: (6/33 = 2, 6/42 = 6, 6/50 = 1)) at the HF/6-31G(d) level of theory.
MD analysis details
All analyses were performed with the cpptraj49 module of Amber22 program package41. The backbone root-mean-square deviation (RMSD) analysis was performed using the backbone atoms of all models with respect to initial structure before MD simulation. The distance criteria for H-bond analysis were defined as ≤ 3.2 Å based on heavy atom distances (acceptor to donor heavy atom), and the angle cutoff is 135°. The contact percentage (%) in H-bond analysis is defined as the percentage of total contacts of the residues throughout simulations. The analysis was performed among active site residues, namely Ser61-Lys64, Ser122-Asn124, Leu158, Lys219-Asp222, and all enzyme residues including tripeptides. Clustering analysis was performed using the HierAgglo algorithm (10 clusters, linkage, based on the Cα RMSD of enzyme residues in order to present representative structure in visualization. Sieve (50, random) was also utilized50. “C” denotes to the cluster. Graphs was plotted by Gnuplot (version 5.4.4)51, ChimeraX52 and PyMOL (version 2.5.4)53 were used for the visualization and illustration of the studied models.