Chemicals
Claramine and spermine (Sigma-Aldrich, MO, USA) were synthesised as a trifluoroacetate salt (for claramine) at a purity >98% as measured by high-performance liquid chromatography (HPLC), stored as a lyophilized powder, and solubilised in DMSO (100%) to a final concentration of 10âmM. Molecules were stored at â80â°C and thawed once before each experiment.
Expression and purification of α-synuclein
The wild-type and cysteine (A90C) variants of α-synuclein were expressed using E. coli BL21 (DE3)-gold competent cells (Agilent Technologies) expressing the pT7-7 plasmid encoding α-synuclein. The expression of α-synuclein was induced by the addition of 1âmM isopropyl β-d-1-thiogalactopyranoside (IPTG). Cells were subsequently centrifuged, lysed by sonication, denatured by boiling before the precipitation of α-synuclein using ammonium sulphate. Following this, protein pellets were dialysed and purified through ion exchange and size exclusion chromatography into 50âmM trisaminomethane-hydrochloride (Tris-HCl) pH 7.4 buffer. All buffers used in the dialysis and purification of the α-synuclein A90C cysteine variant contained 1âmM dithiothreitol (DTT) to prevent the formation of disulfide bonds. The final protein concentration was measured using ultraviolet-visible (UV-vis) spectroscopy on a Cary 100 system (Agilent Technologies). All proteins were aliquoted, flash-frozen in liquid nitrogen, stored at â80â°C and thawed once before each experiment.
α-Synuclein labelling
The A90C α-synuclein was labelled with 1.5-fold molar excess of C5 maleimide-linked Alexa Fluor 647 (Invitrogen Life Technologies) overnight at 4â°C under constant gentle stirring. The unbound dye was removed using Amicon Ultra-15 Centrifugal Filter Units and buffer exchanged into 50âmM Tris-HCl at pH 7.4 by size exclusion chromatography. The final protein concentration was measured using ultraviolet-visible (UV-vis) spectroscopy on a Cary 100 system (Agilent Technologies). All proteins were aliquoted, flash-frozen in liquid nitrogen, stored at â80â°C and thawed once before each experiment.
α-Synuclein phase separation assay
To induce condensate formation, non-labelled wild-type α-synuclein was mixed with the A90C variant labelled with Alexa Fluor 647 at a 100:1 molar ratio in either 50âmM Tris-HCl pH 7.4 and 10% polyethylene glycol 10,000 (PEG) (Thermo Fisher Scientific) by volume at room temperature (20â22â°C). Additionally, claramine, dimethyl sulfoxide (1% DMSO), spermine, NaCl and preformed fibrils were added to the protein phase separation assay for various experiments. 10âµL of the final mixture was pipetted on a 35âmm glass bottom dish (P35G-1.5-20-C, MatTek Life Sciences) and immediately imaged on a Leica Stellaris Will inverted confocal microscope using a 40Ã/1.3 HC PL Apo CS2 oil objective (Leica Microsystems). The excitation wavelength was set to 633ânm for all experiments. All images were processed and analysed in ImageJ (NIH).
α-Synuclein aggregation assay within condensates
Thioflavin T (ThT) 20âµM (Sigma), claramine, 1% DMSO and preformed fibrils, depending on the experiments, were mixed with monomeric wild-type α-synuclein, containing 1 molar % A90C α-synuclein labelled with Alexa Fluor 647 prior to each experiment. The assay mixture, which included 50âmM Tris-HCl, pH 7.4, 10% PEG 10,000, was pipetted onto a 35âmm glass bottom dish and imaged on a Leica Stellaris Will inverted confocal microscope using a 40âÃâ/1.3 HC PL Apo CS2 oil objective. The excitation wavelength 633ânm and 488ânm were used for Alexa Fluor 647 labelled α-synuclein and ThT, respectively. Images were acquired every min for ~40âmin. Images were processed on ImageJ: an area adjacent to the edge of the droplet was cropped and analysed, thus allowing for time-dependent measurement of amyloid formation at the droplets.
α-Synuclein preformed fibril seeds preparation
α-Synuclein pre-formed fibrils were formed from recombinant α-synuclein wild-type monomers diluted in buffer (50âmM Tris-HCl at pH 7.4) to concentrations of ~500âµM. Monomers in were incubated at 40â°C, with constant stirring speed (1,500ârpm) with a PTFE micro stirrer bar and left to aggregate on an RCT Basic Heat Plate (RCT Basic, model no. 0003810002; IKA, Staufen, Germany) for up to 72âh. Samples were centrifuged at 4â°C at 18,800âg for 15âmin. Once fibrils were isolated from supernatant, they were suspended in buffer equivalent to discarded supernatant. The fibril concentration was measured by dissociating a small aliquot of fibrils in a total solution of 4âM guanidinium hydrochloride (GndHCl). After a 30âmin incubation period, the fibril concentration was measured using UV-vis on a Cary 100 system (Agilent Technologies). The fibrils were aliquoted and stored at room temperature before experimentation. Before each experiment, fibrils were pre-treated by 15âs (15 pulses) sonication at 10% power with 50% duty cycle using a Microtip sonicator (Bandelin Sonopuls HD2070) to disperse lumped fibrils.
Fourier-transform infrared spectroscopy (FTIR)
Product from protein phase separation assay in the presence of DMSO (1%) and claramine (75âµM) upon condensate and amyloid formation were washed off glass slide with 50âmM Tris-HCL. Product was centrifuged at 13,800âg for 10âmin, at room temperature. Supernatant containing soluble α-synuclein was discarded, and pellets were washed twice with H2O. Pellets were resuspended in 3âμL H2O and subjected to attenuated total reflectance (ATR) Fourier transform infrared (FTIR) spectroscopy. 2âμL of solution were applied to a Perkin-Elmer Spectrum100 FTIR with an ATR diamond attachment and dried. Prior to scanning, sample and electronics chambers were purged with a constant flow of dry air. 100 replicate scans were averaged from 4000â800âcmâ1, normalized to amide I intensity (~1630âcmâ1 peak), and second derivatives were taken with 9 points for slope analysis.
Estimation of the α-synuclein concentration required for phase separation in water-in-oil droplets
Fabrication of microfluidic devices: The fabrication process of the microfluidics was taken from a previously established protocol42,45,46. In brief, a soft photolithographic process was used to fabricate the master through which microfluidic devices were made. A 50âμm photoresist (SU-8 3050, MicroChem) was spin-coated onto a silicon wafer. This was soft baked at 95â°C for 3âmin. A film mask was placed on the wafer and the whole system was exposed in UV light to induce polymerization. The wafer was then baked at 95â°C for 30âmin. Finally, the master was placed into a solution of propylene glycol methyl ether acetate (PGMEA, Sigma-Aldrich), which helped in the development process. Elastomer polydimethylsiloxane (PDMS) with curing agent (Sylgard 184, DowCorning, Midland, MI) was mixed at a ratio of 10:1 to fabricate the devices. This mixture was then incubated at 65â°C and cured for a total of 3âh. Once hardened, the PDMS was peeled off the master, and holes were punched into the PDMS, which acted as inlets and outlets. Finally, the PDMS slab was bound to a glass slide by treatment with a plasma bonder (Diener Electronic, Ebhausen, Germany).
Formation and confinement of droplets: neMESYS syringe pumps. Syringe pumps (Cetoni, Korbussen, Germany) were used to control the flow rates within the microchannels. Protein solution was mixed with PEG at a ratio of 1:1 at the first junction. At the second junction, the oil phase, which consisted of fluorinated oil (Fluorinert FC-40, Sigma-Aldrich) and 2% w/w fluorosurfactant (RAN biotechnologies) intersected the aqueous phase resulting in water-in-oil droplets being formed. Following droplet generation, droplets were confined within a microfluidic trap42,45,46. In brief, droplets are directed towards an array of traps whereby once a droplet is driven within the microfluidic confinement, it is unable to escape unless a pressure is applied from the outlet. Droplets were then incubated at room temperature to allow for shrinkage. This resulted in an increase of the local concentration of protein and PEG within the droplets which led to protein phase separation. The water-in-oil droplets and the protein phase separation was monitored using fluorescence microscopy.
Calculation of protein concentration during phase separation: The concentration of the protein was obtained by calculating the ratio of the droplet volume just after trapping (V1) and at the point of phase separation (V2), i.e. at the point at which condensates start appearing within the water-in-oil droplet. By multiplying the initial monomeric protein concertation by the value of this ratio, the actual protein concentration at the point of phase separation could be determined.
Transmission electron microscopy (TEM)
α-Synuclein samples from the ThT-based aggregation assay that contained either DMSO (1%) or claramine (75âµM) were obtained after fibril formation. The obtained sample (5âµL) was deposited on a carbon film of 400 mesh 3âmm copper grid. The grids were washed once with Milli-Q water, then incubated with 1% (w/v) uranyl acetate for 2âmin and washed twice again with Milli-Q water before being air-dried at room temperature. Samples were imaged using a Tecnai G2 transmission electron microscope operating at 80â200âkeV.
Liquid-chromatography mass spectrometry (LC-MS)
Monomeric α-synuclein was diluted in storage buffer to a concentration of 5âμM in the absence and presence of claramine (1:1 protein drug molar ratio). Protein liquid-chromatography mass spectrometry (LC-MS) was performed on a Xevo G2-S TOF mass spectrometer coupled to an Acquity UPC system using an Acquity UPLC BEH300 C4 column. The mobile phase was composed of H2O with 0.1% formic acid (solvent A) and 95% MeCN and H2O with 0.1% formic acid (solvent B) at a flow rate of 0.2âmL/min. The electrospray source was operating with a capillary and cone voltage of 20âkV and 40âC, respectively. Nitrogen was applied as desolvation gas at a total flow rate of 850âL/h. The mass spectra were reconstructed using the MaxEnt algorithm on the MassLynx software according to the manual.
Microscale thermophoresis (MST)
α-Synuclein was spun down at 4â°C and 21,100âg for 20âmin prior to the experiment. A serial dilution of 16 concentrations (15âμL per sample) of claramine was diluted into 50âmM Tris buffer (pH 7.4). To account for residual DMSO, the buffer was supplemented with DMSO accordingly. In addition, 0.2% Tween-20 was added to the buffer to prevent the sticking of the protein to the capillary walls. To each dilution sample, 5âμL of 1.5âμM monomeric α-synuclein (98% wild type; 2% Alexa Fluor 647-labelled) was added. The samples were incubated for 30âmin before the MST measurement was performed. Samples were loaded onto the Monolith NTTM standard capillaries and placed in the sample tray of the Monolith NT.115 instrument. All thermophoresis experiments were performed at 22â°C, 30% red LED intensity and 50% infrared laser (IR) intensity with the laser being on for 30âs per capillary. All analysis was performed using the MO. Screening Analysis Software.
Quenching assay
α-synuclein fibrils were prepared by incubating monomers in a 50âmM Tris-HCl pH 7.4 solution at a concentration of 345âμM. The sample were incubated in an Eppendorf tube under agitation at 1500ârpm at 40â°C for 72âh on a RCT Basic Heat Plate (RCT Basic, model no. 0003810002, IKA, Staufen, Germany). After incubation, solutions containing the fibrils were subjected to centrifugation at 4â°C, 18,800âg for 15âmin. Following centrifugation, the supernatant was discarded, and the fibril precipitate was resuspended in the same volume of buffer to maintain a concentration of 345âμM (monomeric equivalents). The fibrils were sonicated for 15 pulses of 15âs at 10% power with 50% duty cycle using a Microtip sonicator (Bandelin Sonoplus HD2070) to disperse lumped fibrils prior to the experiment. 5âmM stock of claramine was prepared in DMSO and filtered using 0.02âμM syringe filters (Whatman Anotop 10/0.02). Solutions containing 75âμM (monomeric equivalents) of fibrils, 20âμM of ThT, and varying concentrations of claramine (25, 50, and 75âμM) were prepared. The fluorescence intensity of each solution was compared to a control solution consisting of 75âμM of fibrils and 20âμM of ThT. All samples were aliquoted into a 96-well plate, and ThT fluorescence was monitored for 30âmin in a plate reader using an excitation wavelength of 440ânm and an emission wavelength of 480ânm. All experiments were conducted as biological replicates, and the data are presented as meanâ±âSD. Statistical significance between different experimental groups was analyzed using an one-way ANOVA test with multiple comparisons correction, with GraphPad Prism 10 (GraphPad Software) being used for all statistical analyses.
Fluorescence recovery after photobleaching (FRAP)
FRAP experiments were performed on condensates using a Leica Stellaris Will inverted stage scanning confocal microscope. To conduct FRAP experiments, a 63âxâmagnification oil objective (63x/1.4 HC PL Apo CS oil) was used. Bleaching was done using the 647ânm laser at 20% intensity for 2âs following a 2âs pre-beach sequence. Immediate post-beach images were captured at a rate of 1âs per frame for 20âs. Intensity traces of bleached area were background-corrected and normalised to reference signal and FRAP time which is defined by the time to half maximal signal recovery. This was determined using the FRAP wizard system (Leica) on the confocal microscope.
Western blot analysis
Phase separation assay was carried out in glass dish with and without claramine. Samples were extracted from glass dish with 50âmM Tris-HCl and centrifuged 21,100âg for 20âmin to separate the soluble (found in sample supernatant) and insoluble (found in sample pellet) fractions, equal volume of buffer was added to insoluble fraction. Both soluble and insoluble (without boiling) samples were loaded onto a NuPAGE 12% Bis-Tris precast protein gel and ran for 35âmin at 200âV. To transfer protein from the gel to a nitrocellulose membrane, an iBLOT2 dry blotting system was used. The membrane was blocked in PBS with 5% BSA at 4â°C overnight and then washed three times with PBS. The membrane was incubated with an Alexa Fluor 488 anti his tag primary antibody (488 anti-α-synuclein MJFR1 (Abcam, catalogue #ab195025); 1:5000 dilution in PBS with 5% BSA) for 3âh at room temperature, washed five times with PBS with 0.02% tween before imaging.
AmyloFit data analysis
The aggregation of α-synuclein within droplets was monitored as described above. The experimental ThT fluorescence readout was uploaded on the free online platform AmyloFit43 (https://www.amylofit.ch.cam.ac.uk). Next, the software normalised the data to 0% and 100% by averaging the values at the baseline and the plateau of the reaction. Upon data normalisation, the concentration of aggregate mass could be observed as a function of time. The minimum and maximum fluorescence intensity, and the aggregation half-time were calculated from the time at which half the protein that is present, initially as monomer, has aggregated, i.e., the time at which the normalized intensity reaches 0.5. A basin-hopping algorithm was applied to fit the experimental data to a model of protein aggregation. Each experiment was repeated four times and then averaged before fitting, while a primary nucleation dominated model was assumed. The number of basin hops was set to 40 for each fit. The applied model was only considered suitable if it was able to match the experimental data well. The AmyloFit user manual can be consulted for more in-depth information on the fitting procedure43.
Cell culture
Human SH-SY5Y neuroblastoma cells were cultured in DMEM/F-12, GlutaMAX (Thermo Fisher Scientific) with 10% foetal bovine serum (FBS) on 75âcm2 cell culture bottles (Greiner Bio-One) at 37â°C and 5% CO2 and split at 80% confluency.
MTT cell viability assay
Cell viability was measured using the 3-(4,5-dimethylthiazol-2-yl)â2,5-diphenyltetrazolium bromide (MTT) assay. α-Synuclein aggregates were prepared on a Corning 96-well Microplate. The fibrils were recovered, collected by centrifugation at 18,625âg for 20âmin at room temperature and resuspended in fresh buffer (50âmM Tris-HCl, pH 7.4). Cells were seeded on 96-well plates (Greiner Bio-One) at 10000 cells/well in 100âµL medium. After 24âh at 37â°C, the medium was discarded and cells were treated in quintuplicates either with fresh medium (medium control), 20% (v/v) 50âmM Tris-HCl, pH 7.4, in fresh medium (buffer control), or α-synuclein aggregates at 20% (v/v) 50âmM Tris-HCl, pH 7.4, in fresh medium. After another 24âh at 37â°C, medium was discarded, and cells were incubated with MTT (Abcam) diluted 1:10 in RPMI medium (Thermo Fisher Scientific) for 4âh at 37â°C. The solution was discarded, and the formazan product was solubilised at 500ârpm and 37â°C for 15âmin in 100âµL cell lysis buffer per well (Abcam) on a PHMP Grant-Bio Thermoshaker. Absorbance at 570ânm was measured on a CLARIOStar plate reader (BMG Labtech), and cell viability was calculated respective to medium control.
C. elegans strains and maintenance
The C. elegans AM134 ((rmIs126[P(unc-54)Q0::YFP]), (YFP)) strain was the control strain used in this study, other strain used was, α-synuclein transgenic strain OW40 (zgIs15 [P(unc-54)αsyn::YFP]), in which α-synuclein is expressed in the body wall muscle cells and fused to YFP8,41. Synchronised axenic nematodes were acquired by bleaching gravid hermaphrodite adult nematodes with sodium hyochlorite. Axenised eggs were resuspended in minimal media buffer (M9) (0.3% KH2PO4, 0.6% Na2HPO4, 0.5% NaCl and 1âmM/L MgSO4), incubated overnight at 20â°C to hatch. Nematodes were maintained on OP50 Escherichia coli bacterial strain seeded on nematode growth medium (NGM) (0.3% NaCl, 0.75% casein, 1âmM MgSO4, 1âmM CaCl2, 250âmM KH2PO4 (pH 6) and 5âµg/mL mM cholesterol) agar (1.7%) plates. For experimental purposes, 5-fluoro-2âdeoxyuridine (FUDR) (75âµM) were added to agar plates to inhibit generation of offspring. 1% of DMSO was seeded onto bacteria lawn of seeded plates for control and 5âµM of claramine was added to experimental plates. Experiments were conducted with L4 stage nematodes at 20â°C and plates were incubated at 20â°C.
Confocal microscopy inclusions quantification in C. elegans
At indicated time points, nematodes were washed with M9, and palleted in 1âmL of M9 solution containing anaesthetic levamisole (10âmM) (Sigma) to induce paralysis. Nematodes were mounted onto a glass Petri plate (MatTek) with the aid of CyGELTM (Biosatus, Ltd), a thermoreversible hydrogel which is liquid when cold and a gel when warmed at room temperature. High-magnification images were acquired with a Leica TCS SP5 inverted confocal microscope scope with an 20x/1.3 HC PL Apo CS2 oil objective. YFP was detected using 512ânm as excitation and an emission range from 549â550ânm. At least 4 nematodes were imaged per condition. Representative confocal images of worms displaying the head (between the tip of nose and the pharyngeal bulb) were analysed using Fiji47. Inclusions were defined as having an area >10 square pixels (0.2 μm/pixel) and a circularity of 0.5-1.
Automated C. elegans motility assay
L4 nematodes were cultured at 20â°C on either DMSO (1%) or claramine (5âµM), motility assay was carried out on NGM agar plates, at indicated time points, and the nematodes were washed off the plates with M9 buffer and spread over an unseeded NGM agar plate, after which their movements were recorded at 20âfps, using a lab-developed microscopic setup between 30âs to 1âmin. At least 100 nematodes were counted in each experiment unless stated otherwise. Videos were analysed using a custom-made tracking code.
C. elegans fluorescence recovery after photobleaching (FRAP)
FRAP experiments were performed on nematodes at indicated timepoints using a Leica Stellaris Will inverted stage scanning confocal microscope. To conduct FRAP experiments, a 63x magnification oil objective (63x/1.4 HC PL Apo CS oil) was used. Nematodes were paralysed and immobilised using levamisole (10âmM) and CyGEL respectively on a glass bottom petri plate. Bleaching was done using the 488ânm laser at 20% intensity for 5âs following a 2âs pre-bleach sequence. Immediate post-bleach images were captured at ~1300âms per frame rate, for ~25âs. Intensity traces of bleached area were background corrected and normalised to reference signal and FRAP time which is defined by the time to half maximal signal recovery. This was determined using the Leica TCS Stellaris FRAP wizard system on the confocal microscope.
Statistics and reproducibility
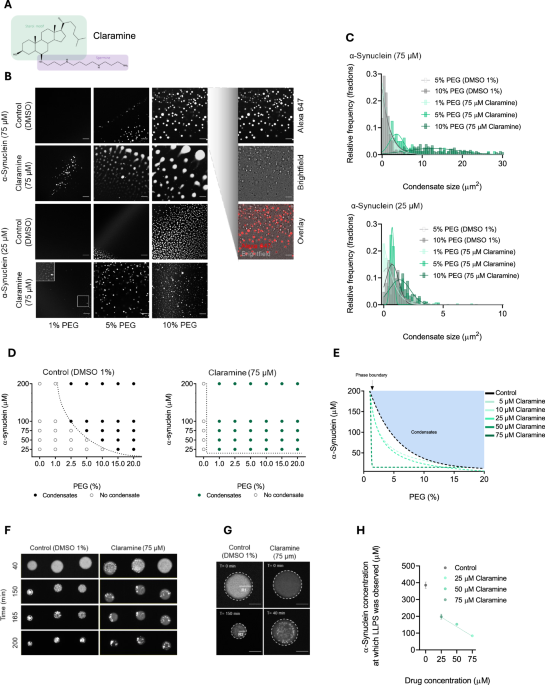
Figure 1B displays representative images illustrating condensate formation under varying PEG and α-synuclein concentrations under both control and claramine conditions. This experiment was conducted at least three times to ensure reproducibility (the corresponding data for Fig. 1D, E, as well as Supplementary Fig. 2, can be found in the source file). This also applies to Supplementary Fig. 3B, where the replicates are detailed in the source file for Supplementary Fig. 3C. Additionally, the experiment presented in Fig. 2D was replicated four times, aligning with the experiments depicted in Fig. 2B, C. Samples were collected after each biological replicate, consistently yielding similar results. In summary, all experiments in this study was repeated at least three times.
Reporting summary
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article.