Materials
Glucose, yeast extract, peptone, ethanol, agar, citric acid, tryptone, fructose, mannitol, NH4Cl, (NH4)2SO4, Na2HPO4âÃâ12H2O, K2HPO4, MgSO4, NaCl, CaCO3, and nisin were used to prepare the culture media, and for the analytical tests. All media components were purchased from Oxoid (Hampshire, UK). Antibiotics (penicillin, ampicillin, tetracycline, and chloramphenicol) were purchased from Hangzhou Microbial Reagent Co. Ltd. (Hangzhou, China). All other reagents used were analytical or microbiological grade and available from local companies.
Microbial strains
A strain of K. hansenii, ATCC 53582, was used for BC production. Escherichia coli and Pseudomonas aeruginosa from the culture collection of the Department of Biology, College of Science, University of Baghdad, Iraq, were used to determine the antibacterial activity of the composite membrane.
Media
A mannitol yeast extract agar (MYA), HS agar media, and their modifications (culture media, broth fermentation media, and production media) were used for BC production. Each medium was tailored to meet specific nutritional requirements and facilitate the desired metabolic activities of the organisms under study. Each medium was prepared according to established protocols and sterilized using appropriate techniques to maintain integrity and prevent contamination.
Plant material collection statement
Plant material collection adhered to institutional, national, and international regulations. Vicia faba is not classified as a protected or endangered species among wild plants in Iraq, hence necessitating no permissions for the research material collection.
Isolation and characterization of the microorganisms
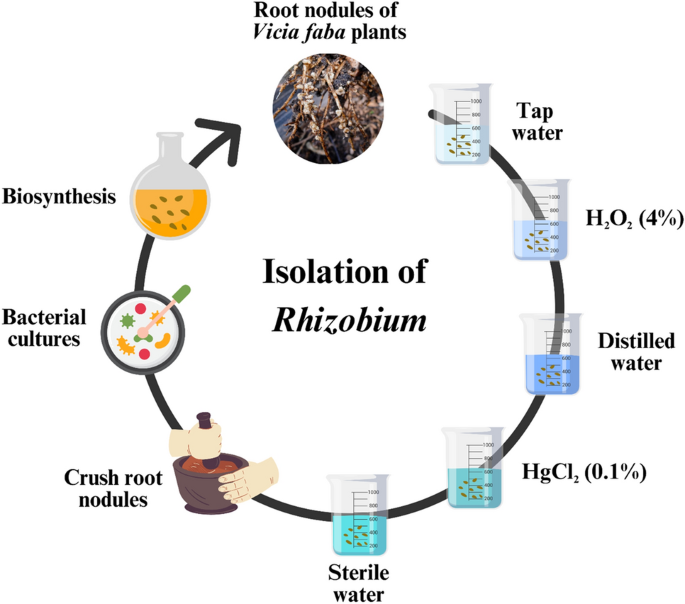
The root nodules of V. faba plants were gathered from various farms in Aladamya, Baghdad city, Iraq, and placed in a sterile container. Healthy root nodules were carefully chosen, subjected to tap water to eliminate soil adhesion, and cleaned with distilled water. Sterilization was carried out using 95% ethanol and H2O2 (4%) for a duration of 2 min, followed by maceration and a subsequent wash with sterile water for 3 min. Finally, the nodules underwent immersion in a 0.1% HgCl2 solution for 2 min to establish a pure culture of Rhizobium sp. (Fig. 1).
Isolation procedures of Rhizobium sp.
Morphological identification of Rhizobium sp.
The extracted sample was taken from the suspension and placed on a glass slide for morphological identification and microscopic examination. Their shapes, cell forms, and morphological characteristics were studied using the Gram stain kit. The examination was conducted under a compound light microscope with an oil-immersion lens at 100Ãâmagnification. Simultaneously, culture characteristics were assessed by inoculating the suspension on MYA and allowing it to incubate at 25 °C for 3â5 days. The standard culture media consisted of 10 g mannitol, 1 g yeast extract, 0.5 g K2HPO4, 0.2 g MgSO4, 0.1 g NaCl, 0.1 g CaCO3, and 15 g/L agar, with an initial pH of 6.8. The resulting solution was transferred to a glass bottle with a 1-L capacity and autoclaved at 121 °C for 15 min42,43.
The collected samples were stored in sterile containers at 4 °C and underwent homogenization and serial dilution. Each dilution was spread on HS agar media (20 g/L glucose, 5 g/L yeast extract, 5 g/L peptone, 6.8 g/L Na2HPO4âÃâ12H2O, 1.5 g/L citric acid, and 20 g/L agar, with an initial pH of 6.0), and incubated for 3 days at 30 °C. After incubation, a single colony was chosen, inoculated into 100 mL/250 mL (100 mL culture media within the 250 mL flask) HS broth media for seed culture, and incubated for 48 h at 30 °C. For BC production, 2% (v/v) of the strained broth was inoculated into 300 mL/500 mL (300 mL culture media within the 500 mL flask) HS broth fermentation media. The microbial cultures were then incubated in treated molasses prepared with 250 mL culture media within the 500 mL flask for 7 days at 30 °C under static conditions. Subsequently, colonies displaying Gram-negative staining and producing white cellulose pellets were selected for further analyses44.
Biochemical identification of Rhizobium sp.
The biochemical tests for the isolate of Rhizobium sp. included various assays performed on bacterial strains isolated from root nodules of the bean under investigation. These tests encompassed the Fluorescence Test45, Catalase Test, Voges-Proskuar Test46, Gelatin Liquefaction Test47, Urease Test48, Citrate Utilization Test, Indol Production Test49, and Cytochrome Oxidase Test50, in addition to assessing bacterial motility, and hydrogen sulfide (H2S) gas production test using SIM media51.
Production and purification of BC
The cellulose-producing capabilities of the isolated Rhizobium sp. and K. hansenii strains were assessed by inoculating flasks with 100 mL of cellulose-producing media and 1 mL of fresh bacterial culture, followed by incubation at 30 °C for one week. The culture media were prepared according to the HS standard: 6.8 g Na2HPO4âÃâ12H2O, 5 g yeast extract, 1.5 g citric acid monohydrate, 5 g peptone, and finally 20.0 g glucose, all homogenized in 1 L of distilled water. The pH of the solution was adjusted to 6.0. The solution was then transferred to a 1-L glass bottle and autoclaved at 121 °C for 15 min52.
Cellulose production was assessed based on the appearance of white cellulose pellicles on the surface of the culture media. Cellulose was extracted from the production media by filtering the cellulose pellicles using Whatman filter paper No. 1, followed by thorough washing with distilled water. The extracted cellulose was decontaminated with a 0.5% NaOH solution at 80 °C for 15 min to eliminate microbial cells and media components. The purified cellulose was then rinsed with distilled water, placed in a Petri dish, and dried in an oven at 105 °C for 1â2 h to determine its dry weight53.
Preparation of the BC composite membrane
The BC membrane was freeze-dried and shaped into circular forms with diameters of 1 cm. Nisin dispersions were prepared in 0.5% (v/v) acetic acid to achieve a final concentration of 1.5% (w/v) and stirred at 37 °C for approximately 3 h. The solution was then oven-dried. The resulting solution was filtered through a polyester cloth to eliminate residual insoluble particles. The BC-dried membrane pellicle was soaked in a nisin-acetic acid solution for 48 h at room temperature to produce a BC-nisin membrane. Various nisin concentrations (100, 200, 300, and 400 IU) were blended to create the BC composite membranes, named cellulose-nisin membranes (BC-Nis).
Optimization of BC production by Rhizobium sp.
Determination of the optimal carbon source for BC production
The effect of carbon sources on cellulose production was studied using different substances such as maltose, fructose, and glucose at a concentration of 2% added individually to HS.
Determination of the optimal glucose concentration for BC production
The effects of different glucose concentrations were studied, and the best carbon source in the production media was between 0.5 and 3%.
Determination of the optimal nitrogen source for BC production
The effect of nitrogen sources on cellulose production was studied using different substances such as yeast extract, peptone, tryptone, NH4Cl, and (NH4)2SO4 at a concentration of 0.05% added individually to HS.
Determination of the optimal concentration of yeast extracts for BC production
The effects of different concentrations of yeast extract were studied, and the best source of nitrogen in the production media was found to be between 0.05 and 0.2%.
Environmental conditions
pH value
Media with a pH between 5 and 7 were prepared to determine the optimum pH for cellulose production. The pH of the media is adjusted by adding concentrated hydrochloric acid to lower the pH or sodium hydroxide to raise the pH. Simultaneously, the source and concentration of the optimal carbon source and nitrogen concentration for optimal cellulose production were determined based on the results of the above experiments.
Temperature
The media were incubated at different temperatures from 25 to 35 °C (25 °C, 30 °C, and 35 °C), with a difference of 5 °C from one media to another, and inoculated with bacterial production. The optimal temperature for cellulose production was determined for 7 days, considering the optimal growth conditions from previous experiments.
Aeration
The experiment was conducted in a glass bottle, one filled with air and the other covered with a cotton lid. The following steps prevented contamination of the culture: (1) sterilization of microbiological instruments and media using a convection oven and autoclave; (2) working in a biosafety cabinet; (3) adjusting the incubator temperature to prevent unwanted growth of organisms.
Characterization of BC
Determination of the dry and wet weights of BC
For this purpose, the wet weight of the BC was determined after washing with distilled water. The dry weight was determined using a digital balance (GENIUS, Sartorius, Germany) with an accuracy of 0.001Â g after drying54.
Determination of the thickness of BC membranes
Thickness measurements of the BC membranes were conducted using a digital micrometer (Mitutoyo, no. 293-766; Tokyo, Japan). 10 different points on each BC sample were selected for measurement, and the average values were calculated55.
Characterization of composite membrane FE-SEM analyzes
To examine the structural properties of the pure freeze-dried BC/K.h, and BC/K.h-Nis membranes, as well as BC/Rhiz and BC/Rhiz-Nis membranes, the BC membranes were fractured using liquid nitrogen, attached to a substrate with carbon tape, and coated with a thin layer of gold. The internal microstructure of the BC membranes, with a focus on the impregnation of nisin nanoparticles into the BC matrix, was observed using FE-SEM (Hitachi S-4800 and EDX-350 (Horiba), Tokyo, Japan) at an accelerating voltage of 10Â kV at room temperature.
Fourier Transmission Infra-red Spectroscopy (FTIR) analyzes
FTIR analysis of freeze-dried BC/K.h, BC/K.h-Nis, BC/Rhiz and BC/Rhiz-Nis samples were conducted using a BIO-RAD FTS-7PC FTIR spectrophotometer (Bio-Rad, Cambridge, MA). The experimental conditions for the FTIR measurements were set to a spectral range of 400â4000/cm and a resolution of 0.25/cm.
Additionally, FTIR spectra of the pristine freeze-dried BC, BC/K.h, BC/K.h-Nis, BC/Rhiz and BC/Rhiz-Nis scaffold samples were analyzed using a Perkin-Elmer FTIR spectrophotometer (Perkin-Elmer, Hopkinton, Massachusetts, USA). The experimental conditions for these measurements were also set to a spectral range of 400â4000/cm and a resolution of 0.25/cm. The samples were thoroughly mixed with potassium bromide (KBr) pellets (IR grade; Merck, Germany) to facilitate the analysis. The resulting mixture was then used to obtain IR spectra, which were transferred to a computer for further spectral analysis.
The water holding capacity (WHC) and water release rate (WRR)
The water-holding capacities of BC/K.h, BC/K.h-Nis, BC/Rhiz and BC/Rhiz-Nis composites were assessed using the sieve shaking method. The dried BC sheets were immersed in distilled water for an appropriate period to allow complete swelling. The plates were carefully removed from the storage containers using tweezers. Subsequently, the samples were placed in a sieve and shaken vigorously twice to eliminate any remaining surface water before weighing. The samples were then air-dried at room temperature, and their weights were recorded at various time intervals (45 h). To ensure complete moisture removal, the samples were dried in an oven at 60 °C for 24 h and then dried at room temperature. The water-holding capacity (WHC) of each sample was determined using the following formula56:
$${\mathbf{WHC}} = \frac{{{\mathbf{Mass\, of\, water\, removed\, during\, drying}} \left( {\mathbf{g}} \right)}}{{{\mathbf{Dry\, weight\, of\, cellulose\, sample}} \left( {\mathbf{g}} \right)}}$$
The WRR was determined by measuring the initial weight of the cellulose samples. These samples were stored under ambient conditions for different durations and weighed periodically until a uniform dry weight was obtained1.
Porosity analyzes
The porosity of BC/K.h, BC/K.h-Nis, BC/Rhiz and BC/Rhiz-Nis composites were determined using the previously developed method. To ascertain the porosity of BC, it underwent a 12-h immersion in distilled water at ambient temperature. The porosity percentage was calculated using the following formula57:
$${\mathbf{Porosity}} \left( \% \right) = \frac{{{\mathbf{wet\,}} {\mathbf{weight}} {-} {\mathbf{dry\,}} {\mathbf{weight}}}}{{{\mathbf{wet\,}} {\mathbf{weight}} {-}{\mathbf{weight\,}} {\mathbf{in\,}} {\mathbf{water}}}} \times 100$$
Swelling analyzes
Swelling abilities of BC/K.h, BC/K.h-Nis, BC/Rhiz and BC/Rhiz-Nis composites were also investigated. The membranes were cut into 2âÃâ2 cm square shapes, dried to constant weight, and subsequently subjected to drying until a constant weight was achieved. After this, water elimination occurred, and the membranes were immersed in deionized water at room temperature58. The swelling ratio (SR%) was determined through the application of the following equation:
$${\mathbf{Swelling\,}} {\mathbf{ratio}} \left( \% \right) = \frac{{{\mathbf{Wt}} – {\mathbf{Wd}}}}{{{\mathbf{Wd}}}} 100$$
Wt represents the weight of the hydrogel in its swollen state, and Wd indicates the hydrogelâs weight when in its dried state. Swelling assessments were performed in triplicate. The average results were then calculated according to the method59.
Filtration analyzes
The filterability of BC/K.h, BC/K.h-Nis, BC/Rhiz and BC/Rhiz-Nis composites were assessed by passing 100 mL of distilled water through samples dried at 70 °C for 4â6 h and determining the diameter. The time taken for the water to filter through each sample was determined as previously reported60.
Antibacterial activity of the composite membrane
To investigate the antibacterial activity of cellulose obtained from K. hansenii and Rhizobium sp., samples were treated with different nisin concentrations for 24 h. The zones of inhibition for E. coli and P. aeruginosa, cultivated on nutrient agar, were determined utilizing the disk diffusion method. BC membranes lacking nisin served as the control specimens. Subsequently, the agar plates were incubated at 30 °C for a duration of 48 h. The antibacterial activity was assessed by measuring the diameter of the inhibition zone surrounding the BC composite membrane, and the results were quantified in arbitrary units (mm).
Data analysis
Each measurement for every group was repeated 3 times (Nâ=â3). The data were presented as the mean standard error for both the control and experimental groups. The experimental data were analyzed using IBM SPSS 24.0, and the statistical significance was determined at pââ¤â0.05. The graphs were generated using GraphPad Prism 9.3 (https://www.graphpad-prism.cn/).