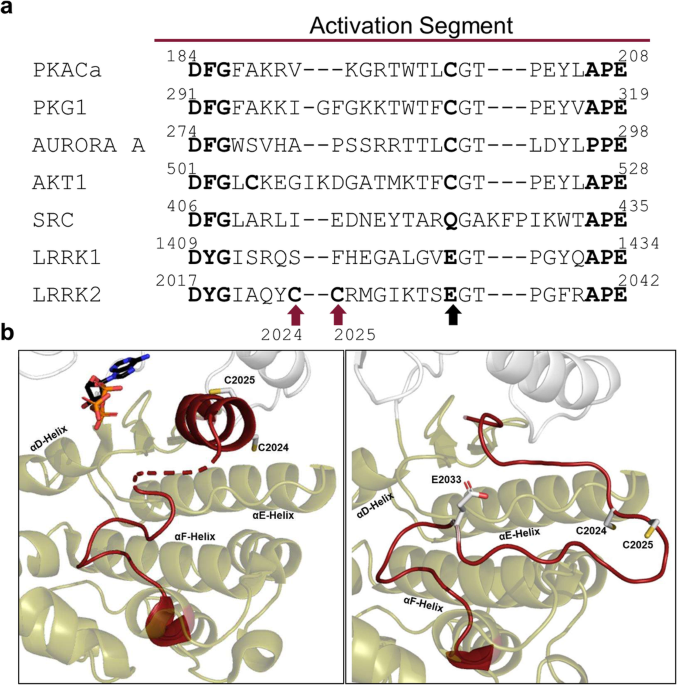
Many eukaryotic protein kinases (ePKs) are known to be regulated in a redox-sensitive manner, and particularly in the Activation Segment (AS), canonical sequences embedding a critical cysteine residue have been identified. A sequence alignment of the AS of selected human protein kinases revealed that in contrast to AGC kinase family members (PKA, PKG1, Aurora A, and AKT1), the canonical cysteine residue (corresponding to C199 in PKA) is not conserved in LRRK2 as a member of the TKL family, where it is a glutamic acid (E2033) (Fig. 1a). Also, in SRC, a member of the Tyrosine Kinase (TK) family, this canonical cysteine is replaced by glutamine. Interestingly, LRRK2 contains two adjacent, highly unique cysteines in the AS, C2024 and C2025. So far, several protein kinases are known to have at least one cysteine at the described positions in LRRK2 (LRRK22024: CLIK1L, TSSK1, TSSK2; LRRK22025: VRK1, VRK2, DYRK4, JNK2, ERK5), but no other kinases have two adjacent cysteines at these positions.
a A sequence alignment of selected human protein kinases revealed two unique cysteines, C2024 and C2025 (red arrows), in the Activation Segment (AS) of LRRK2. The highly conserved region between the DFG/DYGÏ and APE motif (bold) of PKACα, PKG1, AURORA A, AKT1, SRC, LRRK1 and LRRK2 was aligned using CLUSTAL O (1.2.4.). Cys199 in PKA is conserved in many S/T-kinases, but not in SRC, LRRK1 and LRRK2, where it is a glutamic acid (black arrow). b In inactive FL LRRK2 (left, PDB: 7LHW) C2024 and C2025, are located within the helical motif formed by the AS (red). C2025 was directed towards the active site, while C2024 pointed in the opposite direction. The predicted active conformation of LRRK2 (right, AlphaFold2), showed repositioning of C2024 towards surrounding domains.
Two adjacent Cysteine residues change orientation in the LRRK2 kinase domain upon activation
In the full-length (FL) LRRK2, cryo-EM structure (PDB: 7LHW, Supplementary Fig. 1), the kinase domain (KD) is surrounded by the N-terminal and C-terminal domains (NtDs and CtDs), locking the kinase in its inactive conformation31. Various characteristics of an inactive kinase are combined in this FL LRRK2 structure. The highly conserved regulatory (R)-Spine is broken32, the αC-helix is flipped out, and the Y2018 from the DYGÏ motif prevents interaction of E1920 and K1906 with ATP33. Remarkably, the first ten amino acids of the AS (aa2017â2026), including the DYGÏ motif, form a very stable helix31,33, which additionally prevents the kinase from being in an active state. The two adjacent cysteines, C2024 and C2025, are part of this helical motif (Fig. 1b), whereby C2024 in the inactive FL cryo-EM structure is oriented towards the active site cleft, while C2025 points in the opposite direction and is solvent exposed (Fig. 1b left). However, in an active structure of FL LRRK2, predicted by AlphaFold2, the helical motif in the AS is unfolded, and the C2024 and C2025 are reorientated allowing the AL to approach the surrounding COR-B and ROC domains (Fig. 1b right). Additionally, E2033, replacing in LRRK2 the canonical cysteine, is not resolved in the inactive FL cryo-EM structure, while in the active state, predicted by AlphaFold2, E2033 interacts with T2031, one of the putative phospho-sites in the AS (Fig. 1b left).
C2024 and C2025 are critical for redox regulation of LRRK2 kinase activity
To investigate the function of C2024 and C2025 in LRRK2 kinase regulation, we generated LRRK2 Cys-to-Ser substitutions at both positions, whereby serine was chosen as a non-redox sensitive cysteine mimic because of its similar size, and structure. The hyperactive, pathogenic LRRK2 G2019S mutant was used as a control and kinase activity for LRRK2 wt, and mutants were tested in the absence and presence of the highly specific ATP-competitive (type I) LRRK2 kinase inhibitor, MLi-234. Using a microfluidic mobility shift assay (MMSA) with LRRKtide (RLGRDKYKTLRQIRQ) as a peptide substrate, we showed that the C2024S substitution completely abolished kinase activity (Fig. 2a). A commonly used substitution with alanine introduced into C2024 again yielding a kinase inactive protein (Supplementary Fig. 2). In contrast, kinase activity of the C2025S mutant protein was still detectable, however, significantly decreased compared to LRRK2 wt and further inhibited by MLi-2, indicating that the mutant protein can still bind ATP as well as MLi-2. We also tested the ability of LRRK2 wt and the mutant proteins to be auto-phosphorylated on S1292 – a commonly used read-out to analyse LRRK2 kinase activity. Although in MMSA no kinase activity could be detected for C2024S (Fig. 2a), auto-phosphorylation of pS1292 in C2024S protein was dramatically reduced, but still detectable (Fig. 2b, BI), and LRRK2 C2025S protein showed pS1292 auto-phosphorylation comparable to LRRK2 wt (Fig. 2b, BI). For both mutant proteins, C2024S and C2025S, treatment with MLi-2 fully abolished phosphorylation of S1292. In addition, we assayed for trans-phosphorylation of a heterologous substrate, Rab8A on T72, which was significantly decreased for both, LRRK2 C2024S and C2025S compared to LRRK2 wt and again was abolished by using MLi-2 (Fig. 2b, BII). These data indicate the importance of both cysteines within the AS to modulate LRRK2 kinase activity, although both residues may be involved in regulation differently. C2024, in particular, appears to play an important role in a regulatory mechanism of catalytic activity that has not yet been described for LRRK2.
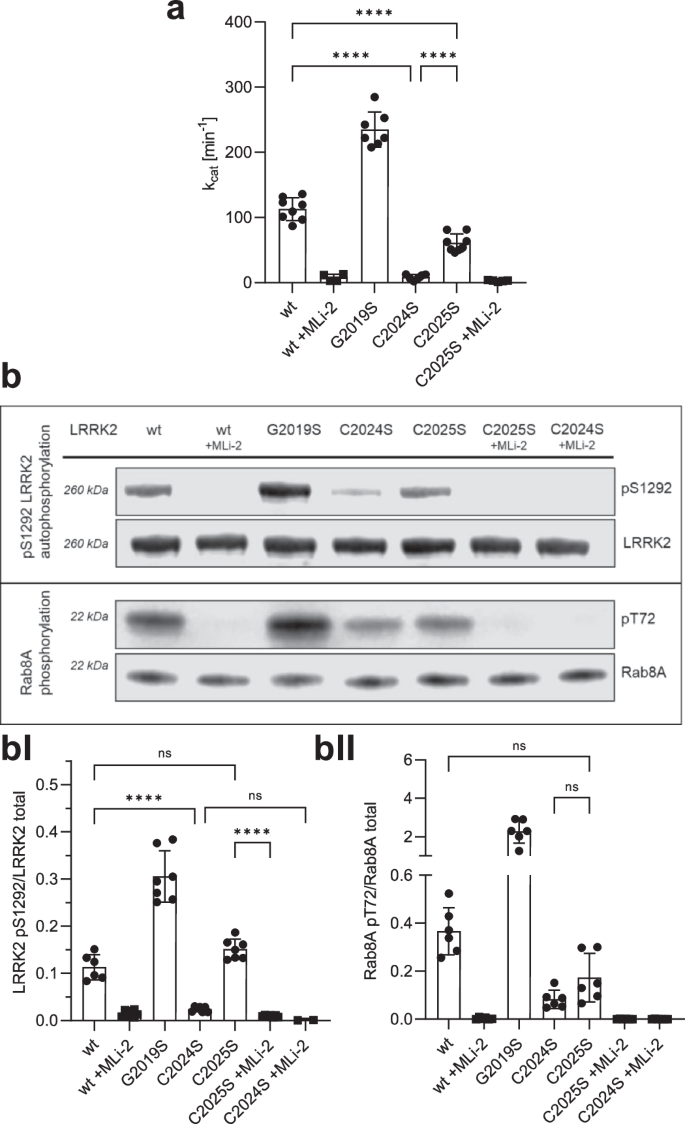
a LRRK2 kinase activity was determined using LRRKtide as a peptide substrate employing a microfluidic mobility shift assay (MMSA). Kinase activity of C2024S was abolished and activity of C2025S was decreased in comparison to LRKK2 wt. b LRRK2 kinase activity was measured either by auto-phosphorylation on S1292 using specific antibodies against pS1292-LRRK2 (Abcam MJFR-19â7â8; upper blot, quantification bI) or by Rab8A phosphorylation on T72 (Abcam MJF-R20; lower blot, quantification bII). Both kinase assay showed reduced activity for C2024S, while phosphorylation of C2025S was comparable to LRRK2 wt. The type I kinase inhibitor MLi-2 was used as a control in all assays. Representative Blots are shown, and data points represent the standard deviation (SD) of at least two protein preparations with three independent measurements based on a one-way ANOVA with a multiple comparison n.s.: Pââ¥â0.05; **: Pâ<â0.01; ***: Pâ<â0.001; ****:Pâ<â0.0001.
Mutation of C2024 resembles a kinase-dead phenotype in a MT docking assay
We then used a cell-based assay to evaluate the capacity of LRRK2 wild-type and cysteine mutations to form filaments that correlate with docking onto microtubules (MT). It has been previously shown that some of the familial PD-mutations of LRRK2 dock spontaneously onto microtubules35,36,37,38.
HEK293T cells were transiently transfected with Flag-tagged LRRK2 full-length, wild-type and mutants, and the ability of overexpressed LRRK2 wild-type and mutant proteins to dock onto microtubules in the absence and presence of MLi-2 was assessed as previously well described35. C2024S behaved similar to the D2017A kinase-dead mutant; it showed no docking onto microtubules even in the presence of MLi-2 (Fig. 3a, AI). Both, the C2024S and the D2017A mutations can render the kinase domain inactive and suggest that an inactive open conformation of the kinase domain prevents docking to MTs. This inactive open conformation was captured by modeling a type II inhibitor39. In contrast, C2025S behaved like wt LRRK2; it could dock onto microtubules only in the presence of MLi-2 in ~75% of the cells (Fig. 3a, AI). Upon treatment of the transfected cells with 250âμM H2O2, the ability of wt and C2025S to dock onto microtubules was reduced by over twofold (Fig. 3c). These results are in line with the in vitro experiments.
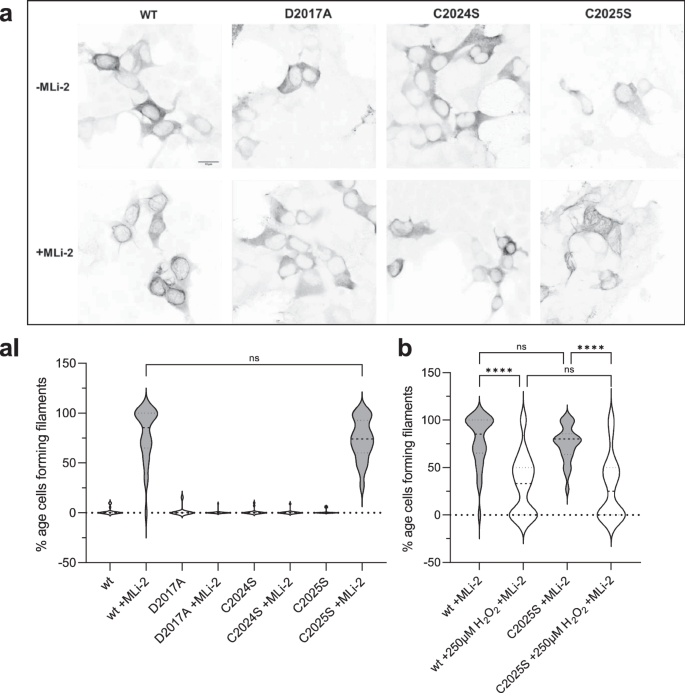
a A filament formation assay was performed in HEK293T cells overexpressing LRRK2 constructs 48âh after transfection. LRRK2 wt in the presence of MLi-2 (100ânM) showed filament formation (80%, aI) of the transfected cells. In the absence of MLi-2 LRRK2 were cytosolically distributed. The kinase dead mutant D2017A was used as a control and is cytosolically distributed in the absence and presence of MLi-2. The C2024S mutant also showed the kinase dead phenotype while the C2025S mutant behaved like the wt (a, aI). b LRRK2 wt and C2025S showed significantly reduced filament formation in the presence and absence of 250âμM H2O2. Representative images, scale bar: 15âμm; (aI, b). Percentages of cells exhibiting LRRK2 filament formation in the absence and presence of MLi-2. Data are shown as violine plots (median and upper and lower quartiles) from three independent sets of experiments based one-way ANOVA with with a multiple comparison n.s.: Pââ¥â0.05; **: Pâ<â0.01; ***: Pâ<â0.001; ****:Pâ<â0.0001.
Reducing and oxidizing agents reversibly modulate LRRK2 kinase activity
Oxidation processes are a common mechanism for regulating the catalytic activity of protein kinases40,41. Because loss of catalytic activity is often due to oxidation of critical sulfur-containing amino acids like cysteines and methionines, reductants like DTT (dithiothreitol) or TCEP (tris(2-carboxyethyl)phosphine) are commonly used in biochemical in vitro kinase assays. To elucidate the potential importance of C2024 and C2025 as redox-sensitive cysteines, we thus asked how different oxidants and reductants affect kinase activity of LRRK2 wt, the pathogenic G2019S mutant, and the C2025S mutant proteins. LRRK2 wt and mutant proteins were treated either with DTT (reductants) or different oxidants (hydrogen peroxide (H2O2), NCA (1-nitrosocyclohexalycetate) or CXL-1020), followed by determination of kinase activity using LRRKtide in a MMSA. NCA and CXL-1020 are two Nitroxyl-(HNO) donors42, which target selectively cysteinyl thiols due to their electrophilic properties, resulting in covalent modifications of cysteines or formation of disulfide bonds.
Recombinant LRRK2 wt and mutant proteins were purified and stored under slightly reducing conditions in the presence of 0.5âmM TCEP (see M&M). Increasing concentrations of DTT were test (Supplementary Fig. 3) and adding 1âmM DTT resulted in a 2.0-fold increase in LRRK2 wt kinase activity, whereas oxidation with H2O2, NCA, and CXL-1020 (250âµM each) significantly inhibited LRRK2 kinase activity in the MMSA (Fig. 4a). The activity of LRRK2 G20219S was also increased in the presence of 1âmM DTT (1.6-fold) (Fig. 4b), and oxidation with all three oxidizing agents significantly inhibited kinase activity. Interestingly, CXL-1020 showed the strongest inhibition on the hyperactive mutant (G2019S, Fig. 4b). LRRK2 C2025S protein showed no activation by reduction with DTT, while oxidation with all three oxidants resulted in inhibition of kinase activity (Fig. 4c). These findings suggest that the ability of LRRK2 to phosphorylate peptide substrates is inhibited by oxidation. Our results suggest that C2024 is unusually reactive and essential for kinase activity. In contrast, C2025, while not essential, may also play a role in modulating redox sensitivity. Only when both cysteines are present maximum activity can be achieved, reflected in conditions using 1âmM DTT. Surprisingly, even though LRRK2 contains a total of 64 cysteines, mutation of a single cysteine (C2024) in the AS essentially abolishes kinase activity, while modification of C2025 significantly alters the activity.
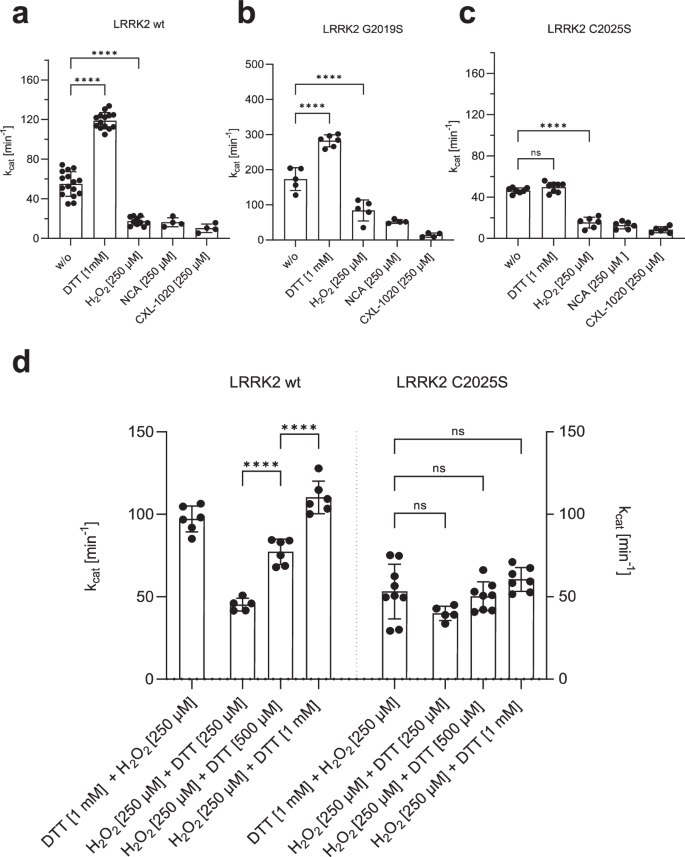
aâc Increased kinase activity towards LRRKtide was determined for both, LRRK2 wt a and G2019S b, after reduction with 1âmM DTT, while activity of C2025S c was unaffected. Oxidation with 250âµM H2O2, NCA or CXL-1020 resulted in reduced activity of LRRK2 wt, G2019S and C2025S. d An experimental setup was approached to test if reversible oxidation of controls LRRK2 kinase activity. First LRRK2 wt and C2025S were preincubated for 20âmin with either H2O2 (250âµM) or DTT (1âmM), followed by incubation for 20âmin with DTT (1âmM) or H2O2 (250âµM, 500âµM, 1âmM). After oxidation with 250âµM H2O2, LRRK2 wt kinase activity was reactivated with DTT in a concentration dependent manner. C2025S showed reduced activity even in the presence of DTT and abolished reactivation with DTT. Data points represent the standard deviation (SD) of at least two protein preparations with three independent measurements based on a one-way ANOVA with a multiple comparison n.s.: Pââ¥â0.05; **: Pâ<â0.01; ***: Pâ<â0.001; ****:Pâ<â0.0001.
Having established that oxidation and reduction have a considerable impact on LRRK2 kinase activity, we asked if oxidation is reversible. LRRK2 wt and C2025S mutant proteins were preincubated for 20âmin either with 250âµM H2O2 or 1âmM DTT, respectively, followed by an incubation for 20âmin with 250âµM, 500âµM and 1âmM DTT or 250âµM H2O2. As shown in Fig. 4d, reduction of LRRK2 wt with DTT is reversible following treatment with H2O2. Adding hydrogen peroxide initially results in slightly decreased kinase activity while treatment with increasing doses of DTT show that oxidation is reversible in a dose-dependent manner (Fig. 4d), as reflected in an DTT-dependent increase in LRRK2 wt kinase activity. For LRRK2 C2025S mutant protein, no effect of either oxidant (H2O2), after previous reduction, or reducing agent could be detected (Fig. 4d). Together, these data indicate that LRRK2 catalytic activity follows a so far undescribed redox-based regulatory mechanism, allowing a switch between an oxidized, âinactiveâ and a reduced âactiveâ state.
Assessing the role of adjacent cysteines in the Activation Loop with MD simulations
Given that the biochemical data supports the importance of C2024 as a reactive cysteine in the AL of LRRK2, we sought to mimic the reactive cysteines computationally. Specifically, we asked if the GaMD simulations would be influenced if C2024 or C2025 were deprotonated. The protonation states of two adjacent histidine residues have been found to be important for the functions of various proteins such as photolyase enzyme (PHR)43, human prion protein (PrP)44, and human forkhead box protein P1 (FoxP1)45. In the FL cryo-EM inactive LRRK2 structure only three residues (aa2028â2030) are disordered33 and the inhibitory helix in the AL locks the kinase into an inactive state. The DYGÏ motif is part of the inhibitory helix, and, based on our earlier GaMD simulations, this helix is very stable (Fig. 5a)31,33. This inhibited conformation remains stable during the simulations (Figure/Video 5B). When the kinase assumes an active conformation, the regulatory triad (E1920, K1906 and D2017) needs to be in place35,46. Specifically, E1920 in the αC-helix should interact with K1906 in β3 sheet when LRRK2 is active. In addition, D2017 in the DYGÏ motif in the C-Lobe should also be close to K1906 in the N-Lobe. In the FL LRRK2 cryo-EM structure, E1920 is far from K1906; however, it is close to Y2018 in the DYGÏ motif (Fig. 5c, d), which is not consistent with an active kinase conformation. In contrast, in the cryo-EM structure, R2026 in the AL is solvent exposed and far from E1920. Based on the MD simulations, however, R2026 is interacting tightly (~ 50%) with E1920 in the αC-helix, which contributes to keeping the αC-helix of the N-Lobe in an âoutâ conformation (Fig. 5e). The simulations also confirmed that E1920 rarely interacts with K1906. In addition, the simulation showed even stronger interaction of the Y2018 hydroxyl with E1920 (~60%). This stable inhibited conformation consisting of Y2018, E1920 and R2026 is similar to what is seen in the inactive conformation of SRC where the DFG motif is in an inactive helical conformation and a basic residue in the AL is interacting with the conserved glutamate in the αC-helix47. In this inhibited state the AL of LRRK2 is constrained and does not approach the COR-B:ROC interface when either C2024 or C2025 is thiolated (R-S–).
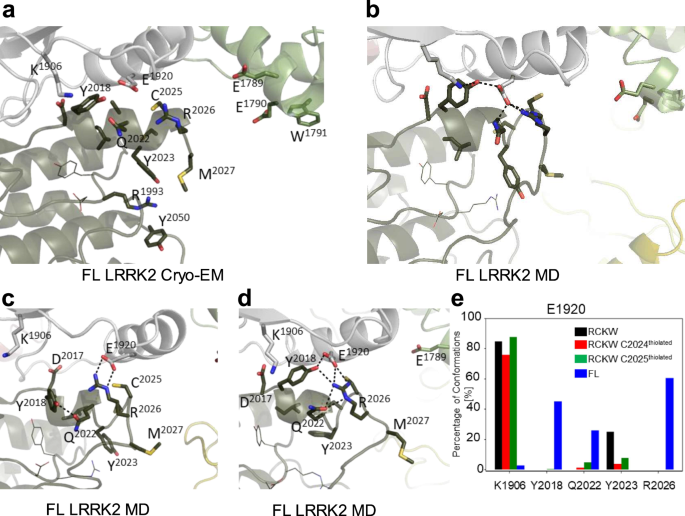
a The cryo-EM structure of inactive full-length (FL) LRRK2 (PDB: 7LHW). The Activation Loop (aa2021â2031) is ordered except for three disordered residues (aa2028-2030). The DY2018G motif through R2026 forms a stable helix that would inhibit kinase activity. b Simulation of FL LRRK2 based on the cryo-EM structure, showing the inhibitory helix (aa2017-2026) and Pâ+â1 loop are stable. c, d Two snapshots that capture different of AL residues with active site residues. e The bar graph quantitates transient interactions of E1920 with K1906, Y2018, Q2022, Y2023 and R2026 during simulations. Simulations were performed with both C2024 and C2025 in the thiolated and non-thiolated form using the RCKW cryo-EM structure (PDB: 6VNO). B MD simulation revealed interactions stabilizing the inactive state of the Activation Loop in FL LRRK2.
In contrast to full-length LRRK2, in the cryo-EM LRRK2RCKW structure48 the inhibitory helix is broken, and the AL (aa2020â2031) is mostly disordered. GaMD simulations of this structure show that the AL does come much closer to the interface between the COR-B helix and the ROC domain where many PD mutations are located. Here, we looked more carefully at the effects of introducing a negative charge on C2024 and C2025. As seen in Fig. 5e, in all the LRRK2RCKW simulations, K1906 is interacting strongly with E1920, which is typical of an active kinase. In this conformation, however, R2026 in the AL now interacts with the COR-B helix, specifically with E1789, which is in close proximity to W1791, a key residue at this interface that interacts with the side chain of the known PD mutation site R1441 (Fig. 6a). In FL LRRK2, R2026 never samples E1789. Surprisingly the frequency of this interaction is also enhanced when C2024 is thiolated (Fig. 6c). In active kinases R1993 in the HRD motif typically interacts with phosphorylated residues in the AL49 (Fig. 6b). We thus asked if the space that R1993 explores is changed when either of the AL cysteines is thiolated. As seen in Fig. 6d, the interaction of R1993 with C2024 is significantly increased when C2024 is thiolated. This is in contrast to the inactive FL LRRK2 where the side chain of R1993 samples many negative charged sites33. The interaction of R2026 with E1797, another residue that is at the ROC:CORB interface, is reduced when C2024 is thiolated but increased when C2025 is thiolated (Fig. 6e). Another key residue that lies between the APE motif and the conserved aspartate (D2055) at the beginning of the αF-helix is Y2050. In fully active kinases with a phosphorylated AL, this side chain is anchored to the HRD arginine (R1993). The interaction of the sidechain of Y2050 with R1993 is also significantly increased when C2024 is thiolated (Fig. 6f). In all other structures so far, including in the cryo-EM of FL LRRK2 (Fig. 5a) Y2050 is pointing away from the HRD arginine.
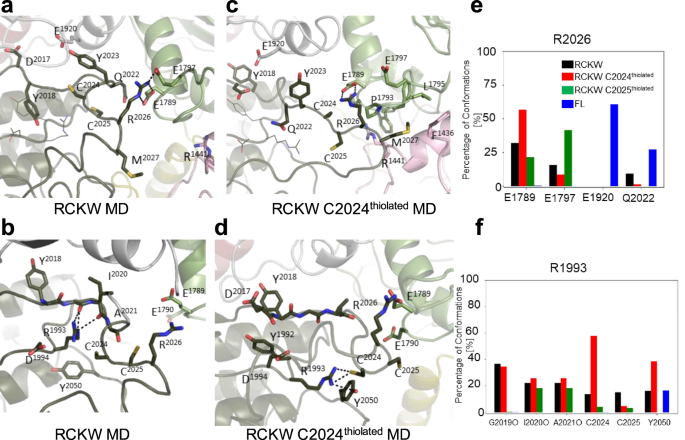
In the RCKW cryo-EM structure (PDB: 6VNO), the Activation Loop (aa2021-2031) was flexible and was thus missing in the model. For the simulations we modeled in the missing residues. aâd Snapshots from MD simulations of this region are shown. For the simulation, we compared FL LRRK2 as well as the RCKW structure with two versions of the RCKW where either C2024 or C2025 were thiolated. a In the RCKW R2026 on the activation loop interacted with E1789 and E1797 in the COR-B domain, b R1993 in the HRD motif interacts with the backbone of G2019 and I2020 in RCKW. c In the simulation of C2024thiolated, the R2026-E1789 interaction increased. d R1993 interacts with C2024 and Y2050. e Bar graph illustrating R2026 interactions seen in the simulations. No interactions were observed between R2026 and COR-B in the inactive FL LRRK2. f Bar graph illustrating R2023 interactions seen in the simulations: Backbone (G2019, I2020, A2021) and sidechain (C2024, C2025, Y2050).
In the LRRK2RCKW simulation, the AL samples space closer to COR-B. As indicated above, R2026 within the AL interacts with both, E1789 and E1797 in COR-B (Fig. 7a). When C2024 is thiolated, the regions beyond R2026 also approach the COR-B:ROC interface more closely. Specifically, M2027 is sometimes found in the space between these two domains (Fig. 7b), whereas in FL LRRK2 (Fig. 5a) this methionine is interacting with other hydrophobic residues in the AS of the kinase domain. When M2027 docks into the interface between the COR-B and ROC domains, it is surrounded by a hydrophobic shell comprised of residues: P1433 and F1436 from the ROC domain, and F1792, P1793, and L1795 from the COR-B domain. M2027 explores this hydrophobic space less when C2025 is thiolated; however, the docking of M2027 into this hydrophobic space is never observed in simulations of the full-length inactive LRRK2 or in the LRRK2RCKW construct (Fig. 7c).
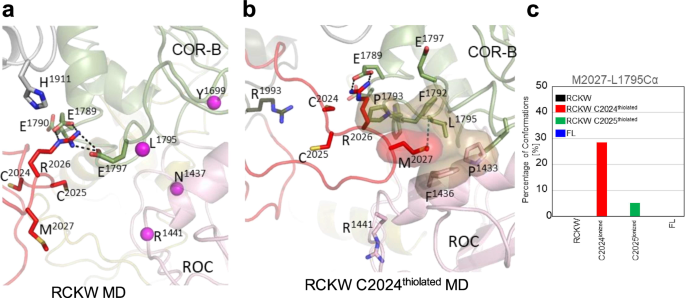
The space between COR-B and the ROC domain is the site of many PD mutations (R1441, N1437, Y1699 and L1795, shown in sphere). a Interactions of R2026 with E1789 and E1797 are captured in the simulations. b A major interaction of M2027 dock into the interface between COR-B/ROC domain is captured when C2024 is thiolated. M2027 is surrounded by hydrophobic residues. c The bar graph illustrates the interaction between Sulfur of M2027 and Cα of L1795 interactions during simulations. The interface shown in b is never explored in the RCKW and FL LRRK2 structures.