Functional reconstitution and structure determination
To investigate the transport activity of TbAQP2 in a simpler manner, we developed the cell-based uptake assay using the fluorescent analog of pentamidine (stilbamidine) as a substrate7. In contrast to uninfected HEK293 cells, those infected with TbAQP2 displayed robust uptake of stilbamidine (~60% positive) within 1âmin (Supplementary Fig. 1a, b). Thus, TbAQP2-dependant uptake of stilbamidine in mammalian cells recapitulates the rapid uptake of pentamidine observed in Trypanosomes.
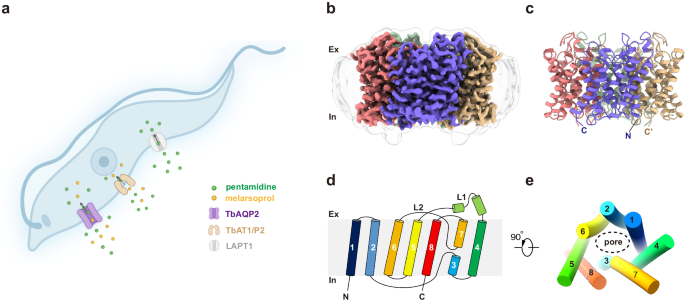
For cryo-EM studies, full-length TbAQP2 was expressed in HEK293 cells, subsequently purified, and reconstituted into lipid nanodiscs (Supplementary Fig. 1câe). We determined high-resolution 3D reconstructions of TbAQP2 alone and in complex with pentamidine or melarsoprol, at overall resolutions ranging from 3.0 to 2.45âà (Supplementary Figs. 2â4). These reconstructions facilitated the building of atomic models with accurate stereochemistry that correlated well with the observed cryo-EM density (Supplementary Table 1). TbAQP2 exhibited the canonical aquaporin fold, featuring eight transmembrane helices (TM1-TM8) connected by six loops (loop A-loop F), and with both the N and C termini positioned on the cytoplasmic side of the membrane (Fig. 1bâe). Four TbAQP2 monomers assembled and formed a tetramer with a fourfold symmetry, closely resembling other aquaporins (Fig. 1bâc).
Conducting pore and selectivity filter
Each monomer of TbAQP2 features a conducting pore that extends ~25âà from the extracellular vestibule to the intracellular vestibule. (Fig. 2aâd). The substrate selectivity of AQPs is thought to be controlled by a selectivity filter (SF) situated below the extracellular vestibule (Fig. 2aâd)22,23,24,25,26. In conventional AQPs, a distinctive feature known as the aromatic/arginine motif (ar/R) in the selectivity filter plays a crucial role in determining selectivity. For channels selective to water, the selectivity filter consists of four highly conserved residues (F58, H182, C191, and R197 in bovine AQP1) (Fig. 2a, e). The histidine residue projects towards the pore, constricting its diameter to less than 2.0âà (Fig. 2a, d, e), effectively preventing the passage of glycerol or other solutes. In glycerol-permeable channels, also known as aquaglyceroporins, the selectivity filter is formed by three bulky residues (W50, F190, and R196 in PfAQP from the malarial parasite Plasmodium falciparum), with the side chain of phenylalanine pointing outward from the pore (Fig. 2b, d, f). This outward orientation relieves the constriction to approximately 3.5âà , allowing the passage of glycerol (Fig. 2b, d, f). TbAQP2, however, deviates from the typical aquaglyceroporin by lacking the ar/R motif found in canonical AQPs and the bulky residues observed in glycerol-permeable PfAQP. (Supplementary Fig. 5). Contrary to conventional AQPs, TbAQP2 features a distinct motif (I110, V249, A259, and L264) in its selectivity filter. This unique motif creates a significantly wider and more hydrophobic selectivity filter, providing steric and hydrophobic environments conducive to the passage of larger organic molecules, such as pentamidine and melarsoprol (Fig. 2c, d, g). Our structural analysis aligns with this observation. Notably, earlier studies demonstrated that mutating these selectivity filter residues to larger counterparts (I110W or L264R) completely abolished pentamidine uptake into trypanosome parasites20.
aâd Channel profiles (aâc) and radii (d) along the pore for BtAQP1 (bovine, PDB: 1J4N), PfAQP (Plasmodium falciparum, PDB: 3C02) and TbAQP2, calculated using the program MOLE, selectivity filter residues are shown as sticks. The channel profiles are colored by lipophilicity generated by ChimeraX: brown means lipophilic; and green means hydrophilic. The regions for SF, NPA motifs, and neck are highlighted in gray, light green, and pink, respectively. eâg SF of BtAQP1 (e), PfAQP (f), and TbAQP2 (g), cross sections of channel profile and key residues are shown. hâj Necks of BtAQP1 (e), PfAQP (f), and TbAQP2 (g), cross sections of channel profile and key residues are shown. k, l Superpositions of TbAQP2 (pink) with PfAQP (yellow), TbAQP2 with BtAQP1 (gray), view from the extracellular side. Arrows indicate the shift of TM6 and loop F (L2-3).
In canonical AQPs, a distinctive âfiremanâs grip-likeâ structure is formed in the middle of the conducting pore by two Asn-Pro-Ala motifs (NPA/NPA) (Supplementary Fig. 6a, b). The conserved asparagine (Asn, N) residues within these motifs play a crucial role in orienting water during channel traversal and preventing proton transport22,23,24,25,26. TbAQP2, on the other hand, exhibits a similar fold with the motifs NS131A/NPS263 (Supplementary Fig. 6c). Interestingly, a hydrogen bond between the hydroxyl group of S263 and N261, not observed in other AQPs, is formed in TbAQP2 (Supplementary Fig. 6aâc). While these residues, S131 and S263, do not directly line the pore through which pentamidine passes (Supplementary Fig. 6c), their role in substrate binding is evident. When these unique NSA/NPS motifs in TbAQP2 are replaced with canonical NPA/NPA motifs, there is a significant ~20-fold decrease in pentamidine uptake, indicating the involvement of these serine (S) residues in substrate binding20. Notably, although S131 and S263 might not directly interact with pentamidine, replacing S263 with alanine disrupts the hydrogen bond to N261, resulting in impaired substrate binding and transport (Supplementary Fig. 6c and Supplementary Movie).
In addition to differences in the selectivity filter and NPA motif, a significant distinction lies in the âneckâ region between the NPA motif and the intracellular vestibule of TbAQP2 compared to other AQPs. The neck of TbAQP2 is notably wider (with a radius of ~2.5âà ) than that of water-specific or glycerol-permeable channels (with radii of ~1.5âà ) (Fig.V). Structural superposition indicates that the expansion of the neck is not caused by alterations in the pore-lining residues but rather by an outward rearrangement of TM5 and the loop connecting TM6 and TM7 (loop F) (Fig. 2k, i and Supplementary Fig. 7d). In canonical AQPs, TM5 and loop F are stabilized by a delicate hydrogen bond network involving highly conserved residues (such as E144, T189, and G190 in BtAQP1 from the bovine, Bos taurus, or E141, T188, and G189 in PfAQP from the malarial parasite Plasmodium falciparum) (Supplementary Fig. 7a, b). However, in TbAQP2, loop F becomes decoupled from TM5, leading to the outward reorganization of loop F and TM5 and, consequently, the expansion of the neck (Supplementary Fig. 7). Overall, these structural differences, including substitutions in residues lining or near the pore, contribute to an expanded selectivity filter and neck in TbAQP2, favoring the binding and subsequent permeation of pentamidine and melarsoprol.
Pentamidine binding
To gain further insights into the mechanism of substrate binding and permeation, cryo-EM studies were conducted on TbAQP2 in the presence of drug substrates. Through optimized sample preparation procedures, structures of the channel bound to pentamidine or melarsoprol were determined at resolutions of 2.45âà . Clear cryo-EM densities inside the conducting pore corresponding to the substrates were identified (Supplementary Fig. 3h). By comparing these structures with the apo-state structure, it was discovered that neither pentamidine nor melarsoprol induces significant conformational changes in the channel (root mean square deviation from the apo-state structure of 0.25âà ² or 0.37âà ², respectively) (Supplementary Fig. 8). Due to their relatively large sizes, only single pentamidine or melarsoprol molecules were observed inside the pore (Fig. 3a, b), in contrast to a line of water or glycerol molecules observed in the pore of canonical AQPs (Fig. 3c), suggesting a distinct permeation mechanism for these antimicrobials.
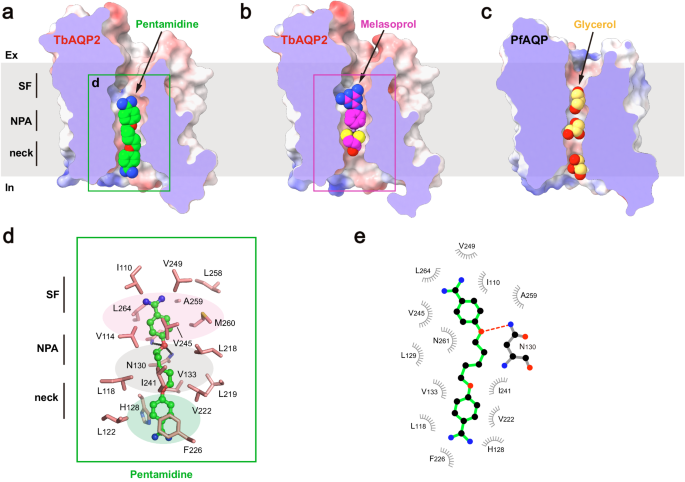
aâc Cross sections of the TbAQP2 and PfAQP, showing pentamidine, melarsoprol, and glycerol (shown as spheres) bound in the conducting pores. d Detailed views of pentamidine bound in the pore. Pentamidine is shown in stickball models, and interacting residues are shown as sticks. e LigPLOT43 diagram of TbAQP2:pentamidine interactions showing hydrogen bond interactions (dashed lines) and hydrophobic contacts ⤠5âà .
Pentamidine was found to be bound in a highly extended conformation, with one of the amidine groups entering the intracellular vestibule of TbAQP2 (Fig. 3a). This suggests that the pentamidine-bound structure represents a pre-entry state. The binding tunnel involves the selectivity filter, NPA motif, and neck. The upper benzamidine moiety of pentamidine was bound in a pocket near the selectivity filter, formed by a series of hydrophobic residues (I110, V114, M260, V245, V249, L258, A259, and L264) (Fig. 3a, d, e). The pentanediol moiety was accommodated by residues surrounding the NPA motif (L118, V133, L218, L219, V222, and I241) (Fig. 3a, d, e). In addition to hydrophobic interactions, the amide groups of N130 and A259 formed hydrogen bonds with the oxygen atom of the pentanediol moiety (Fig. 3d, e). This finding helps explain previous observations that substitutions of the ester groups with thioether or amide groups significantly decrease the uptake efficiency of these compounds20. Furthermore, the lower benzamidine moiety of pentamidine engaged in Ï-stacking interactions with F226 and H128, further contributing to high binding affinity (Fig. 3d, e). Aligning with our structural observations, mutagenesis of these substrate-binding residues resulted in significant decreases in TbAQP2-dependent uptake of stilbamidine (Supplementary Fig. 9).
It is worth mentioning that D265, previously implicated in the direct binding of pentamidine in the âporin-receptorâ mode21, is not within direct bonding distance to pentamidine in our cryo-EM structure. Instead, it forms a salt bridge with a neighboring R269 (Supplementary Fig. 10). This suggests that the reduced pentamidine internalization in the D265A mutant is likely due to an allosteric effect that alters packing, electrostatic, and hydrogen bonding networks critical for maintaining the âfiremanâs gripâ of TbAQP2 and promoting substrate binding and internalization.
Melarsoprol binding
In contrast to the binding site of pentamidine, which was located on the intracellular side of the pore, melarsoprol was accommodated in the middle of the pore (Fig. 3b). To minimize steric clashes, melarsoprol appeared to adopt an approximately planar conformation, with the melamine moiety close to the extracellular side and the dithiasrolane moiety pointing towards the intracellular side (Figs. 3b and 4a, b). The melamine moiety of melarsoprol was bound within the selectivity filter through hydrophobic packing interactions with the pore-lining residues I110, M196, V249, and L264, and a hydrogen bond with the carbonyl group of A259 (FigV). The dithiasrolane moiety of melarsoprol was buried in a flat hydrophobic pocket formed by residues L118, L129, V222, and I241 (Fig. 4a, b).
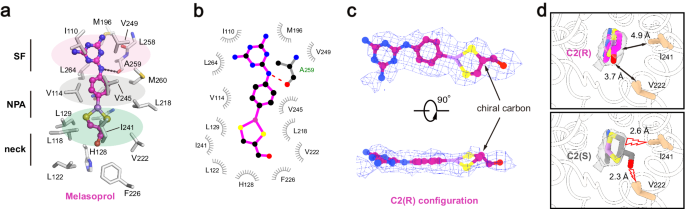
a The detailed view of TbAQP2-melarsoprol interactions. Melarsoprol is shown in ball-and-stick model, and the surrounding residues are shown as sticks. b LigPLOT43 diagram of TbAQP2-melarsoprol interactions, showing the hydrogen bond interactions (dashed lines) and hydrophobic contacts (â¤5âà ). c the bound melarsoprol adopts C2(R) configuration. Cryo-EM densities are shown as meshes, and the chiral cartoon of melarsoprol is highlighted with arrows. d Interactions between the carbinol group of melarsoprol and the surrounding residues of TbAQP2. Model in either C2(R) configuration (upper panel) or C2(S) configuration (lower panel) was docked into the density of melarsoprol (semi-transparent rendering). The distances between carbinol groups and surrounding residues are labeled and highlighted with arrows or lightning plots.
We identified a chiral carbon (C2 atom) in the dithiasrolane moiety of melarsoprol, presenting two possible chiral configurations (Fig. 4c, d). During cryo-EM sample preparation, a mixture of melarsoprol diastereomers was added to the TbAQP2 sample with aâ~20-fold molecular excess (see Methods). The high-quality cryo-EM density inside the pore exhibited excellent correlation with one melarsoprol molecule in the C2(R) configuration (Fig. 4c). To explore the impact of chirality at the C2 atom on melarsoprol binding, we modeled a molecule in the C2(S) configuration into the same binding site (Fig. 4d, lower panel). Structural analysis revealed the emergence of severe steric clashes between the carbinol group of melarsoprol and nearby pore-lining residues (V222 and I241) (Fig. 4d, lower panel). Collectively, these results indicate configuration-selective binding of melarsoprol through TbAQP2.
Molecular dynamic simulations
To explore the permeation pathway of pentamidine, we conducted umbrella sampling (US) simulations to derive free-energy profiles. Initially, steered molecular dynamics simulations (SMD) were employed to pull pentamidine from the binding site in two directions along the conducting pore, mimicking both association and dissociation processes. These simulations generated the necessary windows for the subsequent US simulation. Within the US simulation, a harmonic potential was applied to restrain the ligand in each window, and free-energy profiles were then reconstructed using the Weighted Histogram Analysis Method (WHAM). Our results indicated that the pentamidine binding site corresponds to the minimum free-energy location within the conducting pore (Fig. 5a), consistent with our structural observations. Specifically, our simulations revealed that the energy barrier for pentamidine to exit the channel towards the cytoplasm was approximately 10âkcal/mol, while the barrier to exit towards the extracellular side was around ~14âkcal/mol. Notably, experimental studies have indicated a strong dependence of pentamidine uptake on the membrane potential in trypanosomes (approximately â125âmV for T. brucei)27. To investigate the impact of membrane potential on pentamidine permeation, we conducted simulations in the presence of a negative membrane voltage. Notably, the entry energy barrier towards the cytoplasm decreased to ~5âkcal/mol, while the exit energy barrier towards the extracellular side increased to ~24âkcal/mol (Fig. 5a). These findings suggest that, in the presence of membrane potential, pentamidine has a greater tendency to enter the cytoplasm than to exit the cell. This aligns with experimental observations showing significant accumulation of pentamidine at mM levels inside trypanosome parasites, and no detectable pentamidine efflux when extracellular drug was removed4,28. Our simulations have revealed that in the presence of membrane potential, pentamidine exhibits a notable preference for entering cells over exiting, as indicated by the differences in energy barriers. This distinctive behavior shares some similarities with inwardly rectifying ion channels, such as the mitochondrial calcium uniporter29.
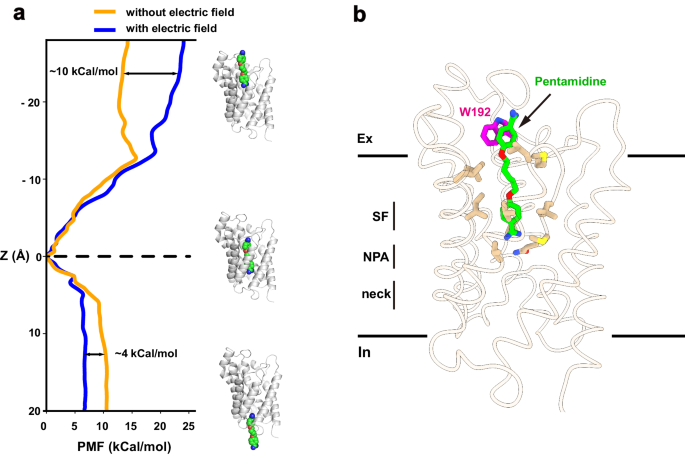
a Free-energy profile of pulling pentamidine to the cytoplasm and the extracellular, from the bound position, with (blue line) or without (yellow line) of an electrical field. b Residue W192 involving pentamidine binding and permeation. Cartoon representation of the local minima on the protein-ligand binding path. Pentamidine (green) and W192 (magentas) were shown in sticks.
Residue W192, situated near the extracellular vestibule of TbAQP2, seems to play a role in the uptake of pentamidine, as evidenced by significantly reduced pentamidine uptake upon mutagenesis20. Despite the absence of direct interactions between pentamidine and W192 in our cryo-EM structure, a detailed analysis of simulation results identified a local minimum free-energy site in the pentamidine permeation route (Fig. 5a, b). In this structural snapshot, pentamidine adopts an extended conformation, with one amidine group entering the selectivity filter (Fig. 5b). The other amidine group is positioned in the extracellular vestibule, forming a pi-pi interaction with W192 (Fig. 5b). To further probe the interaction between W192 and pentamidine, we utilized MM/GBSA simulation to calculate the interaction energies of pentamidine with wild-type TbAQP2 and the W192A mutant, using the local minimum captured in the US simulation as the starting coordinate. The binding energy of the W192A mutant to pentamidine decreased by 4.2â±â0.2âkcal/mol compared to the wild-type protein. Therefore, our calculations suggest that W192 likely serves as a docking site for pentamidine during its permeation through TbAQP2.